
Addition Polymers
Polymers of Class 12
Addition Polymers
Polyolefins:
These are generally obtained from ethylene or its derivatives. The polymerization normally takes place at a temperature between 473−673 K under high pressure and in the presence of traces of oxygen.
Polyethylene or Polyethene:
It is a polymer of ethylene. It is manufactured by heating pure ethylene to 465−485 K under high pressure (1500 − 2000 atm) in the presence of traces of oxygen (0.03 to 0.1%).
nCH2 = CH2 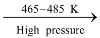 (−CH2 − CH2 −)n
(−CH2 − CH2 −)n
Ethylene Polyethylene:
It is a whitish, translucent polymer of moderate strength and high toughness.
Uses:
Its major uses are as packing films, pipes, containers, laboratory apparatus, bottles, buckets, toys, mould articles and electrical insulators.
It may be noted that these days two types of polythene are used which have widely different properties. These are, low density polythene (LDPE) and high density
polythene (HDPE).
The low density polythene is prepared as discussed above. It consists of highly branched chain molecules. Due to branching, the polythene molecules do not pack well and therefore, it has low density (0.92 g/cm3) and low melting point (384 K). Low density polythene is transparent of moderate tensile strength and high toughness. It is mainly used.
(i) as a packing material in the form of thin plastic film bags.
(ii) for insulating wires and cables.
(iii) in the manufactures of pipes, toys, bottles, etc.
On the other hand, high density polythene is prepared by heating ethylene at about 333−343 K under a pressure of 6 − 7 atm in the presence of a catalyst such as triethylaluminium and titatnium tetrachloride (known as Zeigler Natta catalyst).
n CH2 = CH2 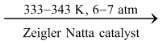 (−CH2 − CH2 −)n
(−CH2 − CH2 −)n
Polyethylene:
This polymer consists of linear chains and therefore, the molecules can get closely packed in space. It has, therefore, high density (0.97 g/cm3), and higher melting point (403 K). It is quite harder, tougher and has greater tensile strength than low density polythene.
It is used in the manufacture of containers, buckets, tubes, pipes, house wears etc.
(ii) Polypropylene or Polypropene:
The monomer unit is propylene. It is manufactured by passing propylene through hexane (an inert solvent) containing Zeigler Natta catalyst (a mixture of triethylaluminium and titanium tetrachloride).
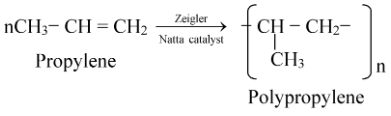
It is harder and stronger than polyethylene.
Uses:
(i) for packing of textiles and foods,
(ii) for manufacturing liners of bags, lining material for TV cabinets and refrigerators,
(iii) for making ropes, fibres, heat shrinkable wraps for records and other articles.
(iv) for making automobile mouldings, seat covers, carpet fibres etc.
Polydienes:
These are polymerized products of alkadienes.
(i) Neoprene or synthetic rubber: Neoprene is a synthetic rubber which resembles natural rubber in its properties. Natural rubber is a polymer of isoprene (2−methyl−1,3−butadiene).
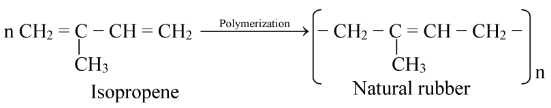
The synthetic rubber, neoprene is obtained by polymerization of chloroprene (2−chloro−1,3−butadiene).
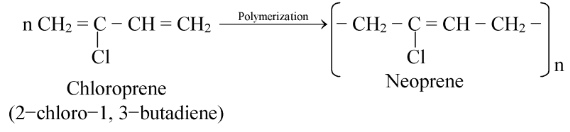
The starting material, chloroprene is obtained by dimerization of acetylene by passing it through aqueous solution of NH4Cl and cuprous chloride at 343 K followed by treatment with HCl.
2HC ≡ CH 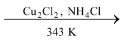 H2C = CH − C ≡ CH
H2C = CH − C ≡ CH 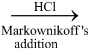 CH2 = CH − CCl = CH2
CH2 = CH − CCl = CH2
Vinyl acetylene Chloroprene
Neoprene is superior to natural rubber in its stability to aerial oxidation and its resistance to oils, gasoline and other solvents.
Uses:
(i) for making belts, hoses, shoe heals, stopper etc.
(ii) in the manufacture of containers for storing petrol, oil and other solvents.
(ii) Buna−S
It is obtained by polymerization of 1,3−butadiene and styrene in the ratio of 3 : 1 in the presence of sodium.
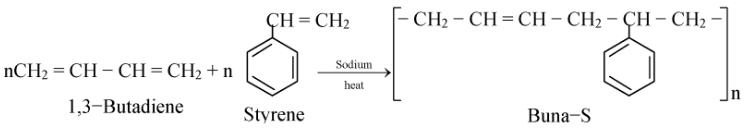
In Buna−S, Bu stands for butadiene, Na for sodium and S stands for styrene. It is also called SBR (Styrene Butadiene Rubber). It has less tensile strength than natural rubber.
Uses:
(i) for making automobile tyres.
(ii) for making rubber soles, belts, hoses etc.
