
Condensation Polymers
Polymers of Class 12
Condensation Polymers
These are formed by the condensation of two or more monomers with the elimination of simple molecules like H2O, NH3, ROH, etc. Some common examples of condensation polymers are:
Formaldehyde resins:
These include polymers like Bakelite and Melamine polymers.
(i) Phenol formaldehyde resins (Bakelite): It is a condensation polymer and is obtained from phenol and formaldehyde in the presence of basic catalyst.
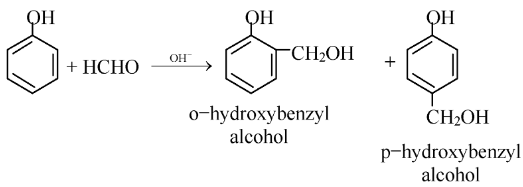
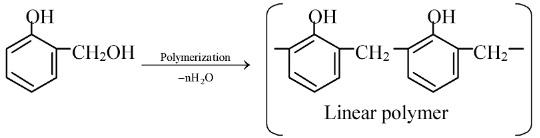
The condensation of o−hydroxybenzyl alcohol or p−hydroxy benzyl alcohol gives a linear polymer.
The ortho and para substituted phenols can undergo polymerization to produce a cross−linked polymer known as bakelite.
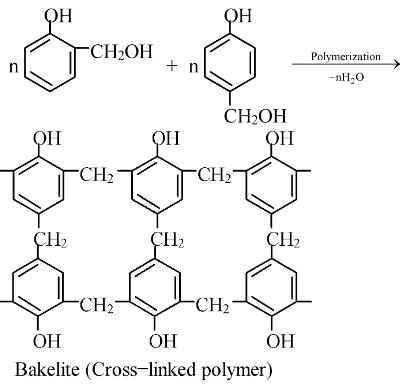
Uses:
Soft bakelites with low degree of polymerization are used for making glue for binding laminated wooden planks and in varnishes. High degree polymerization gives hard bakelites which are used for making combs, fountain pens, barrels, electrical goods, formica table tops and many other products.
(ii) Melamine formaldehyde resin: It is a polymer formed by the condensation of melamine which is a heterocyclic diamine with formaldehyde. The polymerization
occurs as
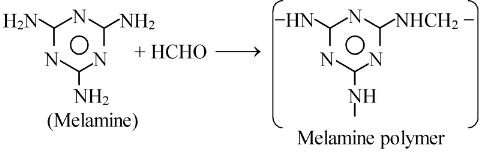
Uses:
It is used in making crockery. These are used for making cups and plates which are quite hard and durable. They do not break on being dropped.
