
Polystyrene Or Styron
Polymers of Class 12
Polystyrene Or Styron
The monomer units are styrene molecules. It is prepared by free radical polymerization of styrene in presence of benzoyl peroxide.
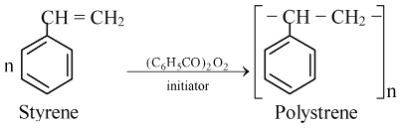
Properties of Polystyrene
Commercial polystyrene is a radically initiated linear atactic polymer. It is amorphous and transparent. The polymer is hard, brittle and transparent at room temperature. It is soluble in aromatic hydrocarbon solvents, cyclohexane and chlorinated hydrocarbons. It sounds like metal when dropped. It decomposes at elevated temperature into a mixture of volatiles, a significant part of which is the monomer which can be identified from its characteristic smell.
Polystyrene, being hydrocarbon in nature, has very low moisture absorption, this feature coupled with its good mouldability, dimensional stability and low moulding shrinkage.
Polystyrene has poor outdoor weather resistance and a tendency to yellowing and crazing on long use. It possesses very good electrical insulation characteristics and a low dielectric loss factor at moderate frequencies.
Uses
It is transparent in nature. It is used for making hot drink cups, toys, combs, house hold articles, tiles to be used in covering ceilings and flowers. Expanded polystyrene finds extensive use in packaging and shock absorbing applications, in thermal insulation and as acoustic improvers in halls and auditoria. Largest outlet of polystyrenes is in the packaging field. High impact grades are suitable for use as toys, games and sports articles, casings and it gives a good electric insulator that is moulded into parts for radios, television sets, automobiles and equipments and inner liners of refrigerators. Another major outlet of polystyrene is in the making of ion−exchange resins.
Nylon−66
Polymer which has polyamide linkage (−CO − NH−). Therefore these polymers are known as nylons. A nylon−66 has hexamethylenediamine and adipic acid as its monomer unit.
nNH2 − (CH2)6 − NH2 + nHOOC − (CH2)4 − COOH
Hexamethylenediamine adipic acid
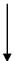
(−CO − NH − (CH2)6 − NH − CO − (CH2)4 − CO −)n
Nylon−66
It is used for making bristles for brushes, in textiles in making sheets. It is blended with wool to make socks and sweaters, cords and climbing ropes.
(a) Nylon−6: Another polymer of this class is nylon−6. It is a monomer of caprolactum which is obtained from cyclohexane.
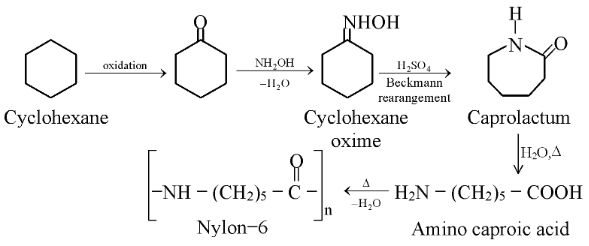
It is used for making tyre cords, fabrics and ropes.
(b) Nylon−6, 10: A polymers of hexamethylene diamine (six carbon atoms) and sebacic acid (ten carbon atoms).
nNH2 − (CH2)6 − NH2 + nCOOH − (CH2)8 − COOH 
hexamethylene diamine sebacic acid
(−CO−(CH2)8−CO−NH−(CH2)6−NH − CO − (CH2)8−CO−)n
nylon−6,10
These polymers are formed by the condensation of two or more monomers with the elimination of simple molecules like H2O, NH3, ROH etc.
Terylene:
It is a condensation polymer which is known as polyester. Terylene is a polymer of ethylene glycol and tetrephthalic acid. It is known as terylene or Dacron.
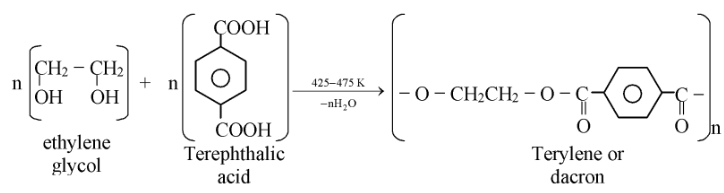
It is a very strong fibre and is used for making cloth by mixing with cotton, magnetic recording tapes, etc.
An important polymer of polyesters class is glyptal. Glyptal is a polymer of ethylene glycol with phthalic acid.
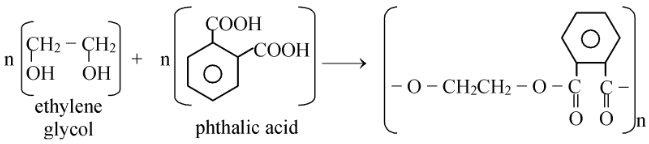
It was commonly used in manufacture of paints, lacquers, building materials such as asbestoss, cement, etc.
Uses
These films are suitable in electrical applications and packaging and as magnetic recording tapes. The fibre is made by a melt spinning process. Poly ethylene terephthalate is the most important synthetic fiber to have found widespread textile applications either alone or more commonly as different blends with cotton or wool. The fibre is widely known as Terylene (ICI trade name) or Dacron (Dupont trade name). The polyester fibres possess good crease resistance and wash and wear properties. A sizable fraction of the polyester fibre is used as the reinforcing cord in the tyre and related industry.
