DALTON’S LAW OF PARTIAL PRESSURE
States of matter of Class 9
DALTON’S LAW OF PARTIAL PRESSURE
According to this law total pressure of a non reacting mixture of gases is equal to the sum of partial pressures of individual gases.
P = PA + PB + PC + ……………….. (in mixture of gases which do not react chemically)
Let nA moles of A, nB moles of B and nC moles of C are filled in a vessel of volume V maintained at temperature T. Then, total pressure will be
PV = (nA + nB + nC)RT (Gas equation) …..(i)
Let only nA moles of A is added in the vessel then
PAV = nART …..(ii)
Similarly,
PBV = nBRT …..(iii)
PCV = nCRT …..(iv)
On adding, (ii), (iii) and (iv) we get
(PA + PB + PC)V = (nA + nB + nC) RT …..(v)
Now comparing equation (i) and (v) we get

Alternatively
Dividing equation (ii) by (i)
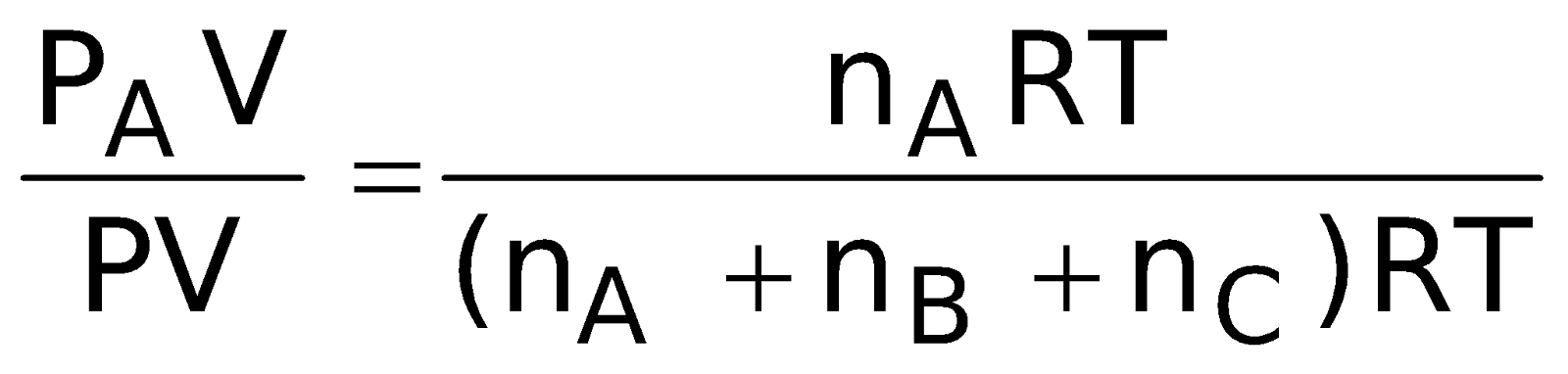
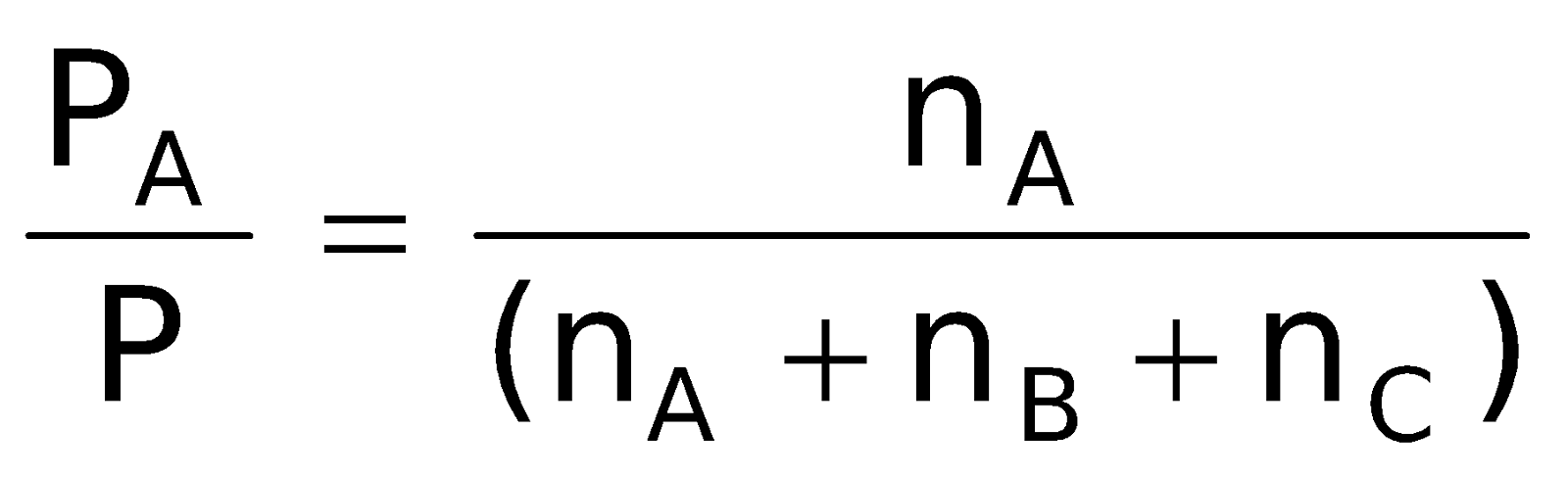 = XA (mole fraction of A)
= XA (mole fraction of A)
PA = PXA …..(i)
Since, PB = PXB …..(ii)
PC = PXC …..(iv)
on adding (i), (ii) and (iii) we get
(PA + PB + PC) = P (XA + XB + XC)
Similarly, XA + XB + XC =1
So, 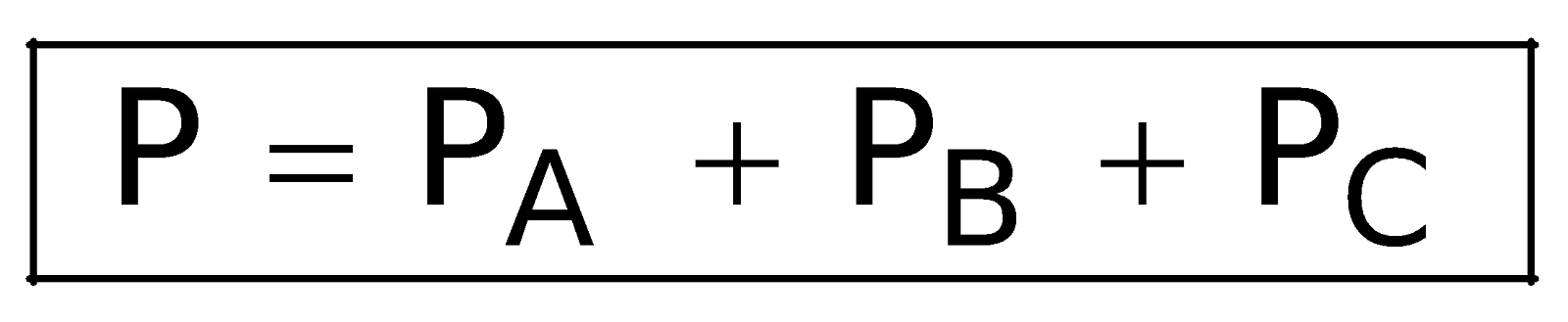
The law can even be applicable to a mixture of reacting gases under the condition when they have attain equilibrium (at which the number of moles of each constituent species remains fixed.
Example6 A 2.5 litre flask contains 0.25 mole each of sulphur dioxide and nitrogen gas at 27°C. Calculate the partial pressure exerted by each gas and also the total pressure.
Solution: Partial pressure of SO2
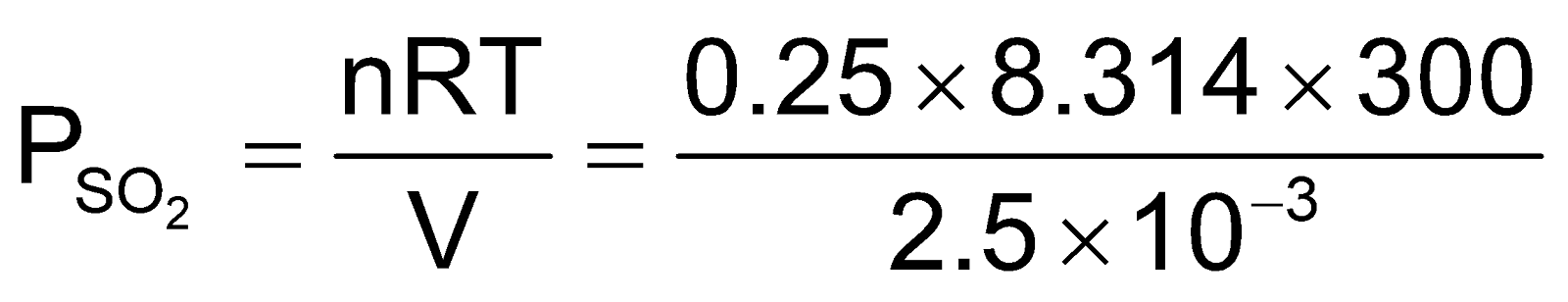
= 2.49 × 105 Nm–2 = 2.49 × 105 Pa
Similarly 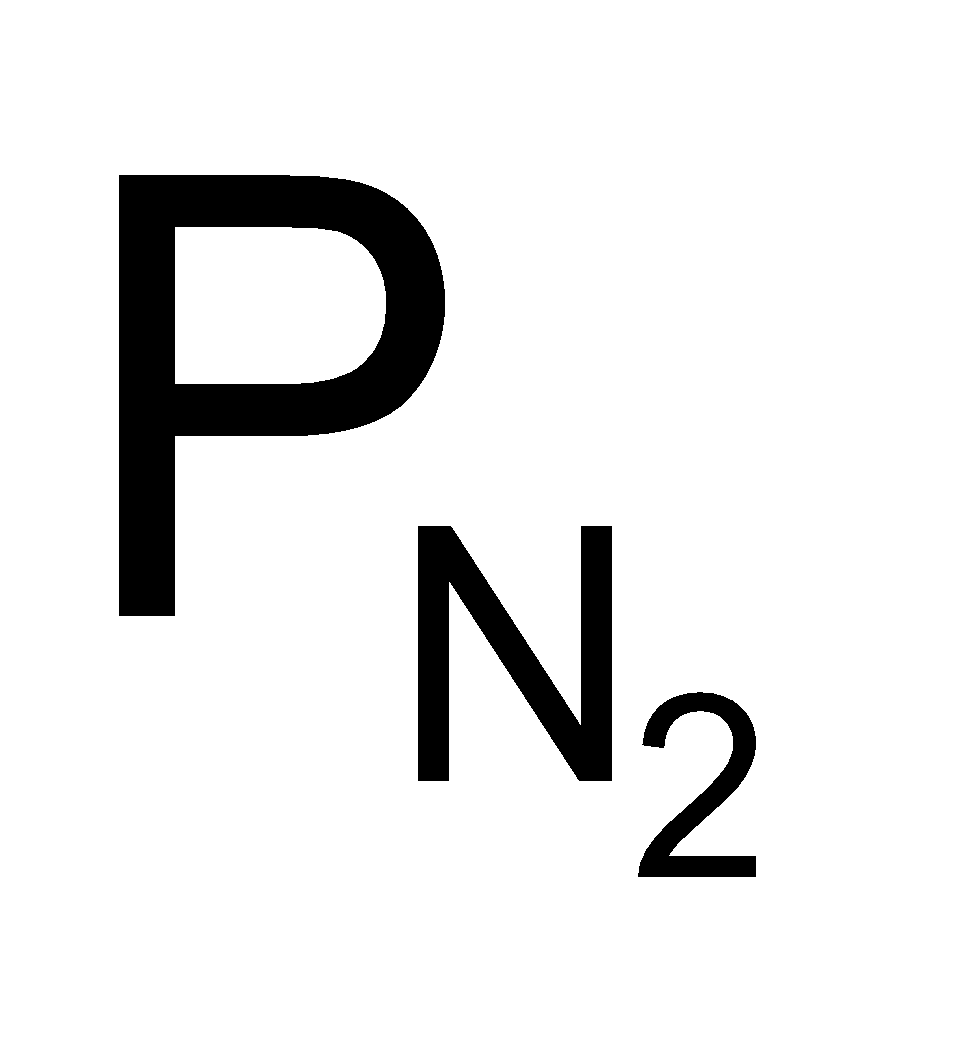 = 2.49 × 105 Pa
= 2.49 × 105 Pa
Following Dalton’s Law
P Total =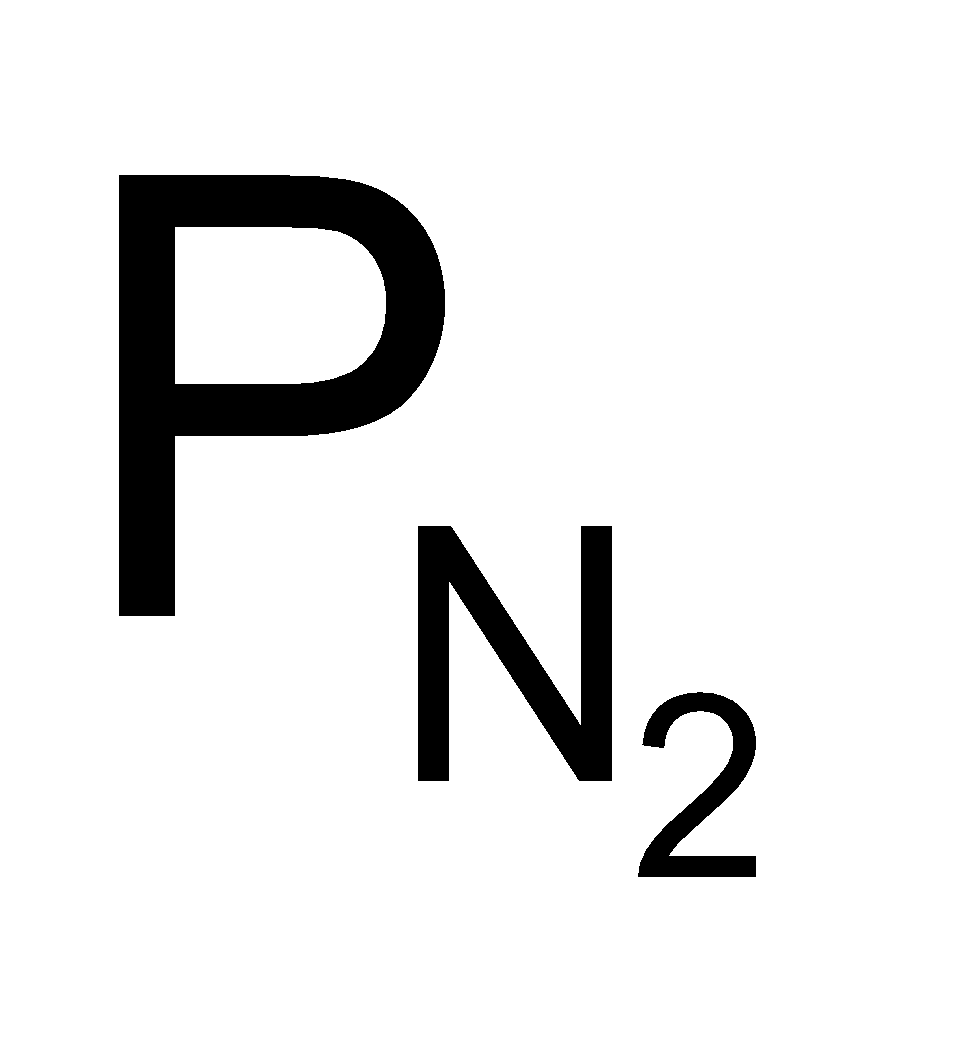 +
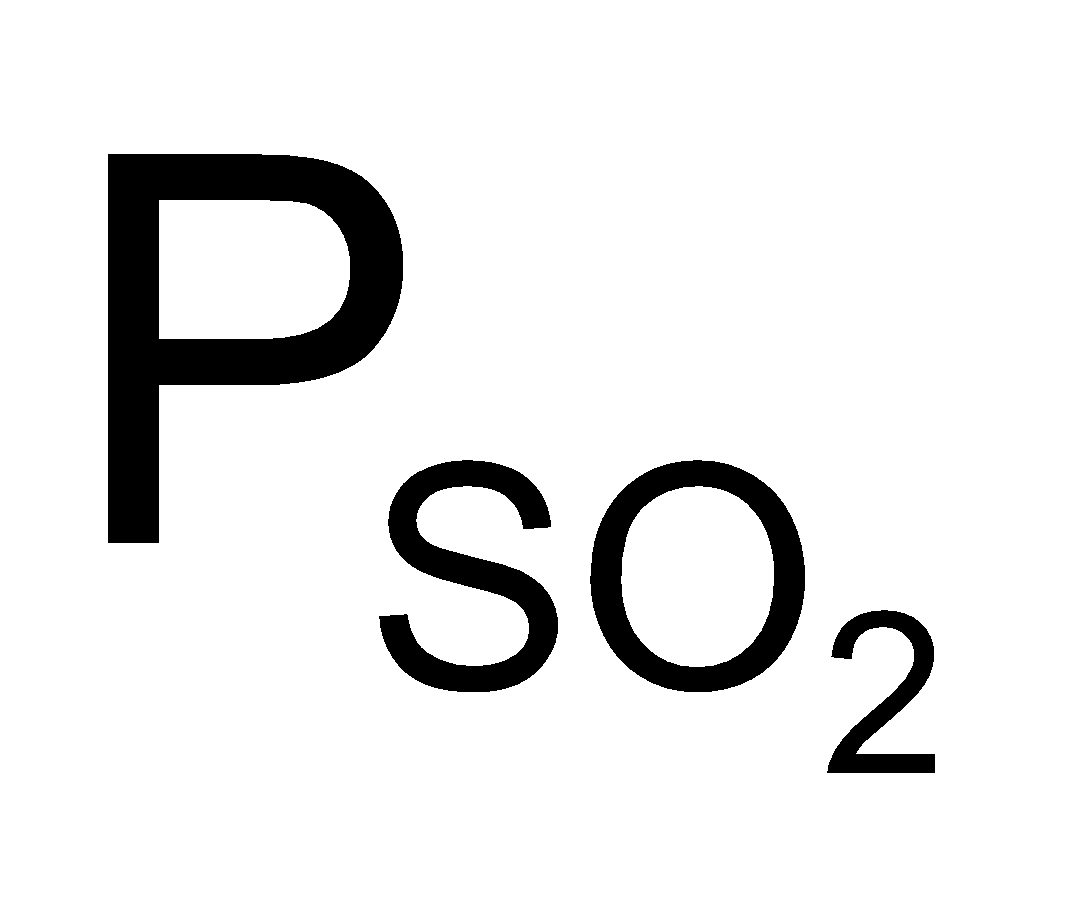
+ 
= 2.49 × 105 Pa + 2.49 × 105 Pa = 4.98 × 105 Pa










