GRAHAM’S LAW OF DIFFUSION/EFFUSION
States of matter of Class 9
GRAHAM’S LAW OF DIFFUSION/EFFUSION
Thomas Graham (1831) studied the rates of diffusion of different gases and observed that lighter gases diffuse at faster rates than the heavier gases. He came out with a generalization called Graham’s law of diffusion/effusion. According to this law.
“Under identical conditions of temperature and pressure, the rate of diffusion/effusion of a gas is inversely proportional to the square of its density (or molecular mass)”.
Mathematically,
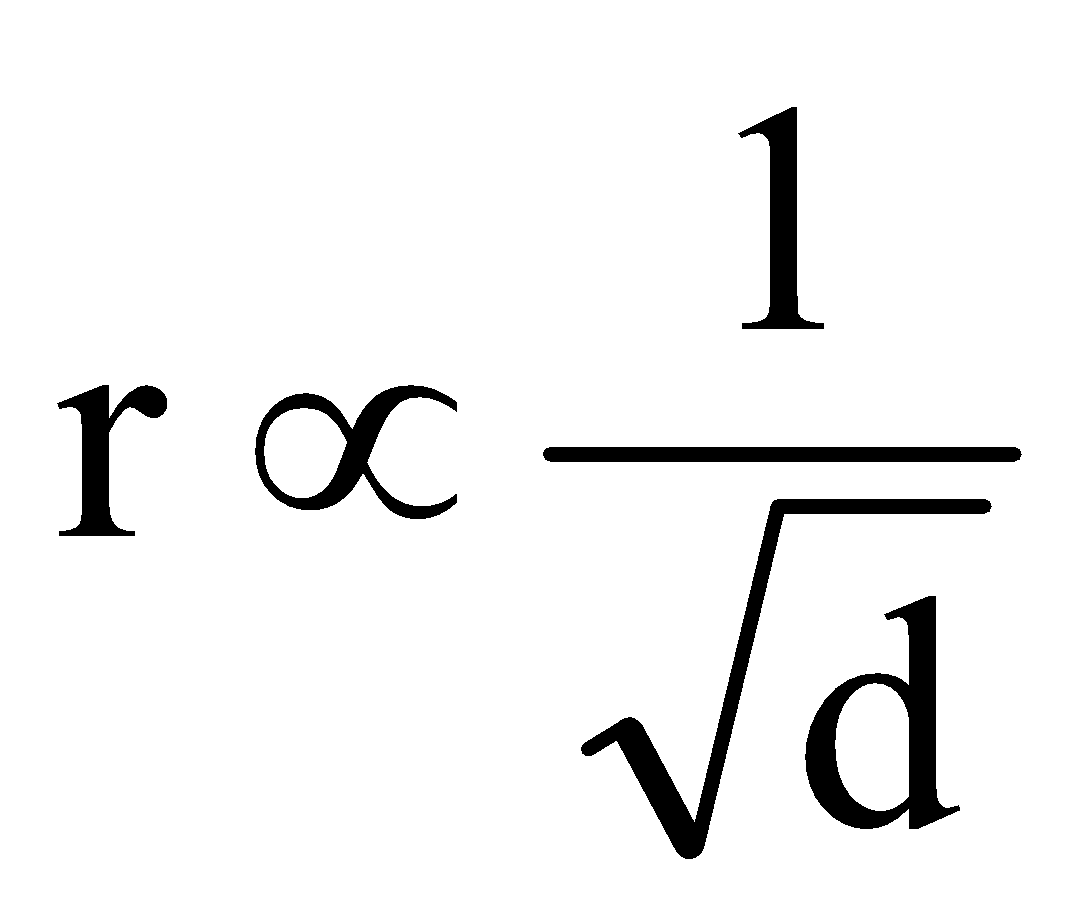 or
or 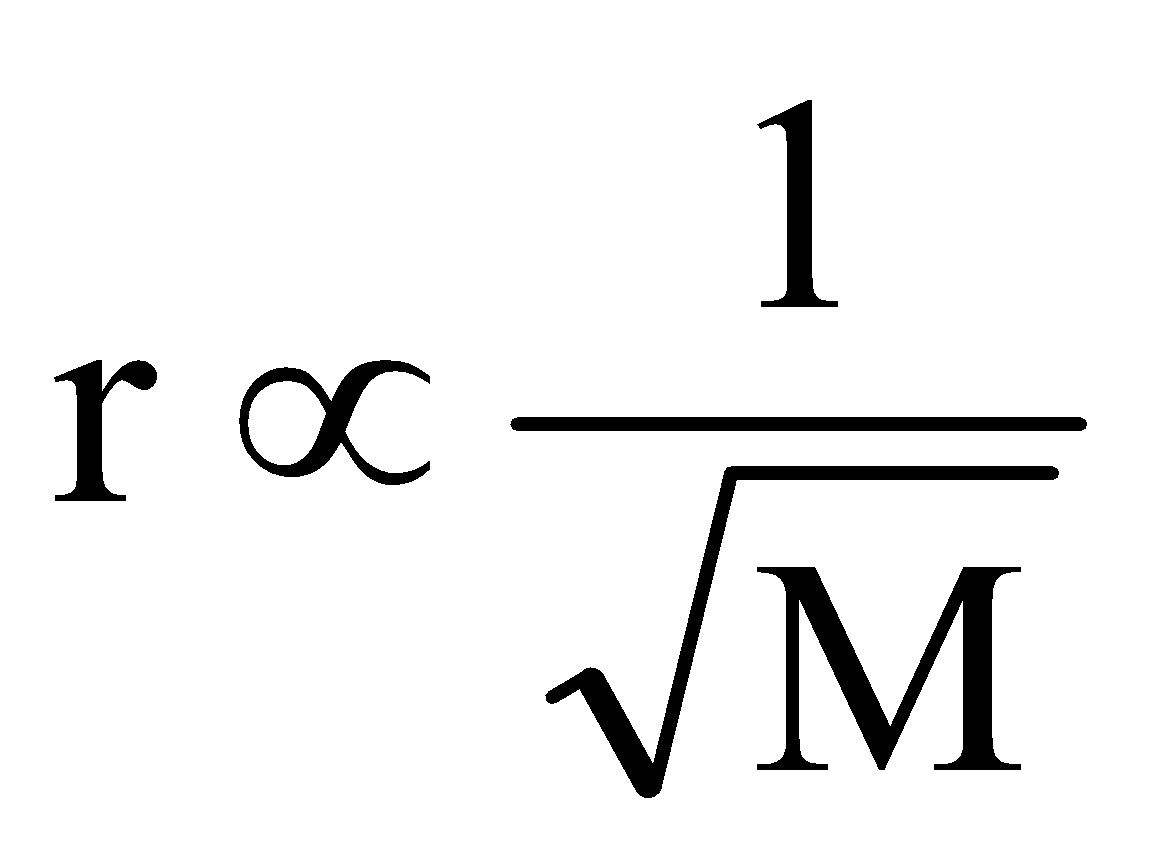 .
.
where, r is rate of diffusion/effusion of gas, d is density of gas and M is molecular mass of gas.
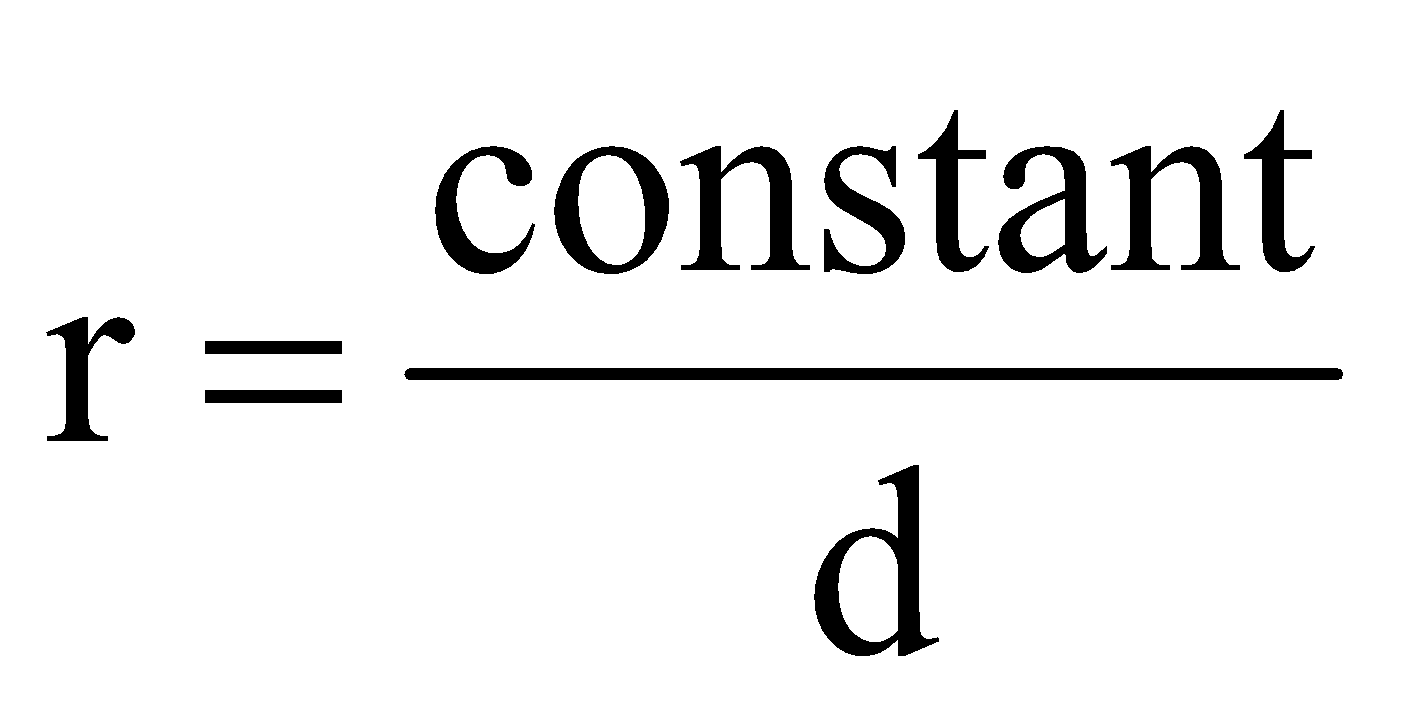 or
or 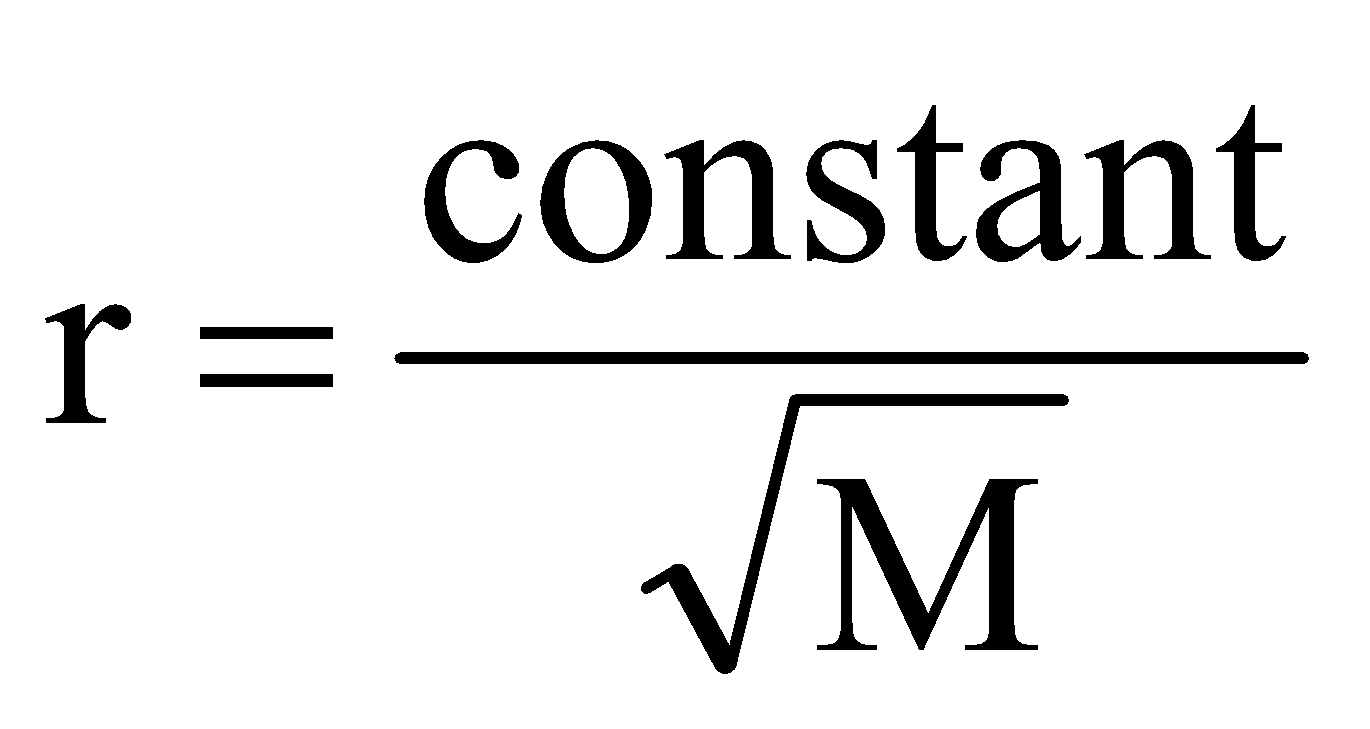 .
.
If r1, d1, M1 and r2, d2, M2 are rates of diffusion/effusion, densities and molecular masses of gas 1 and 2 respectively then, we have,
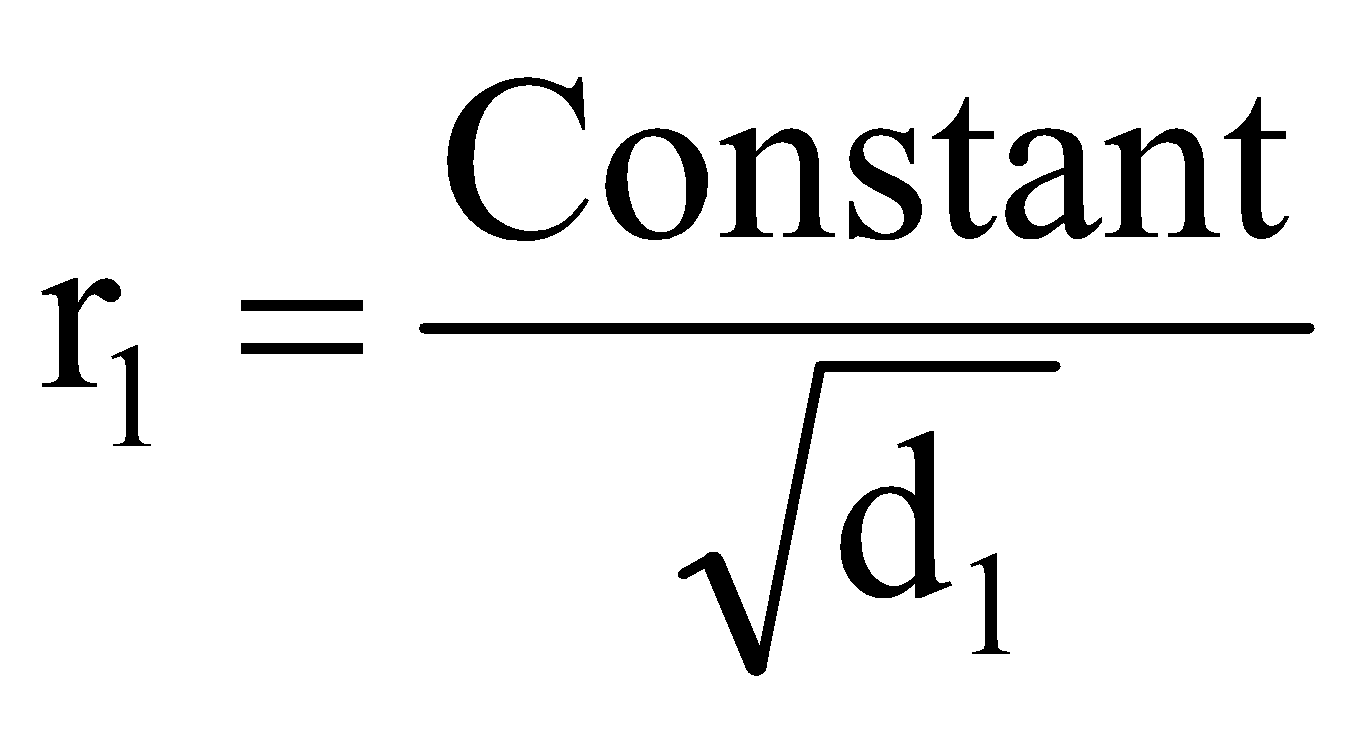 or
or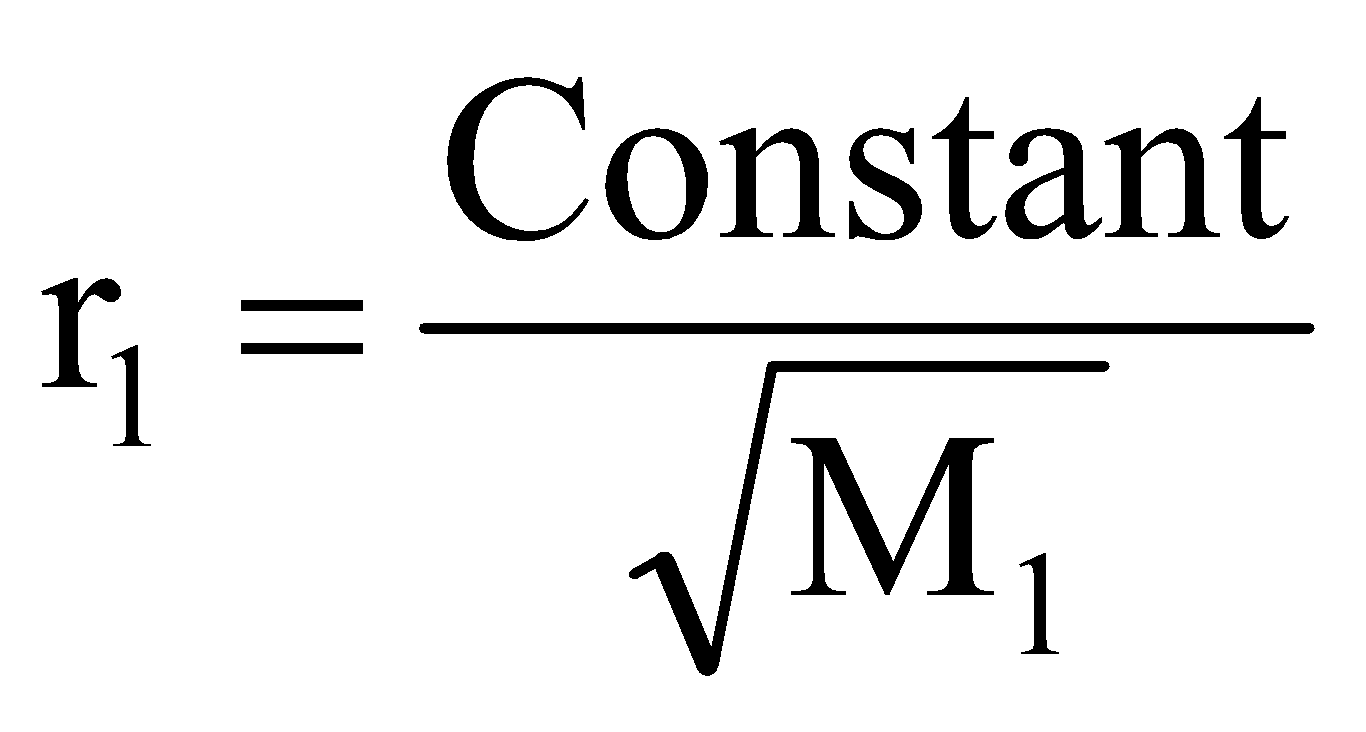 ..... (i)
..... (i)
and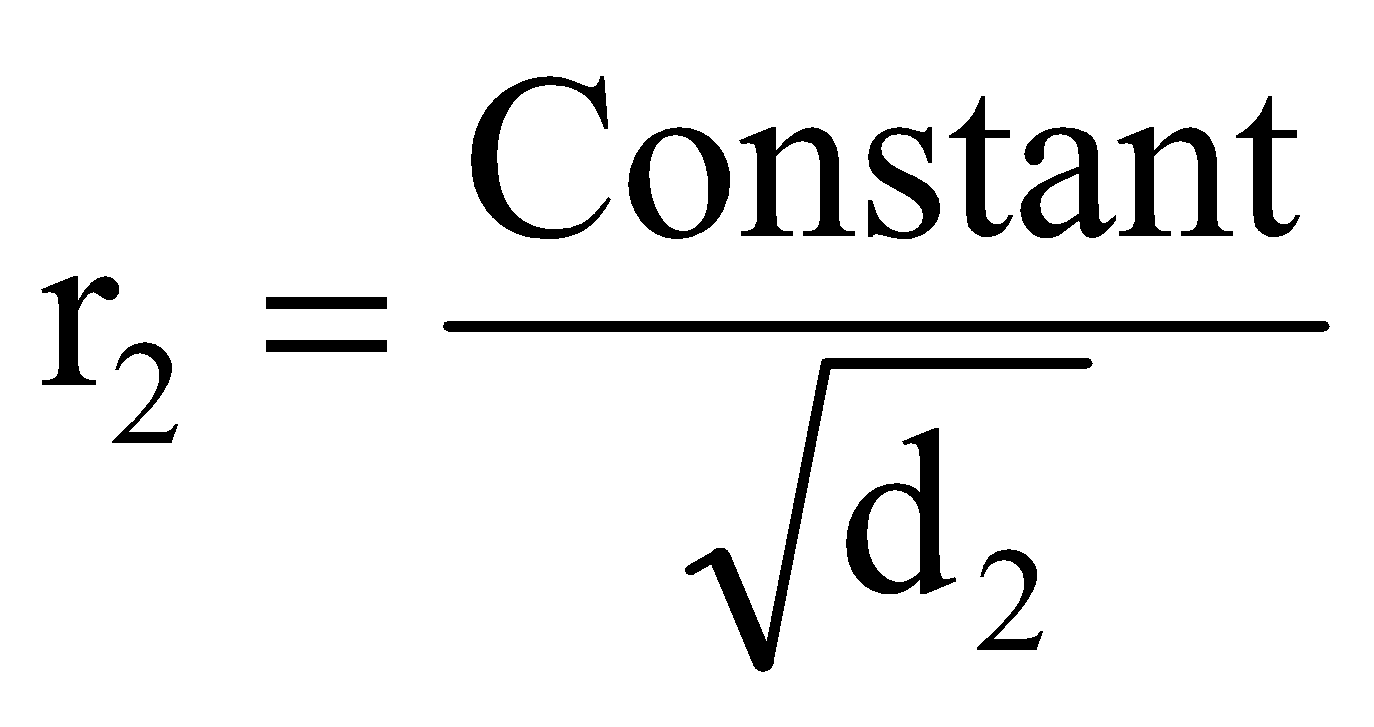 or
or 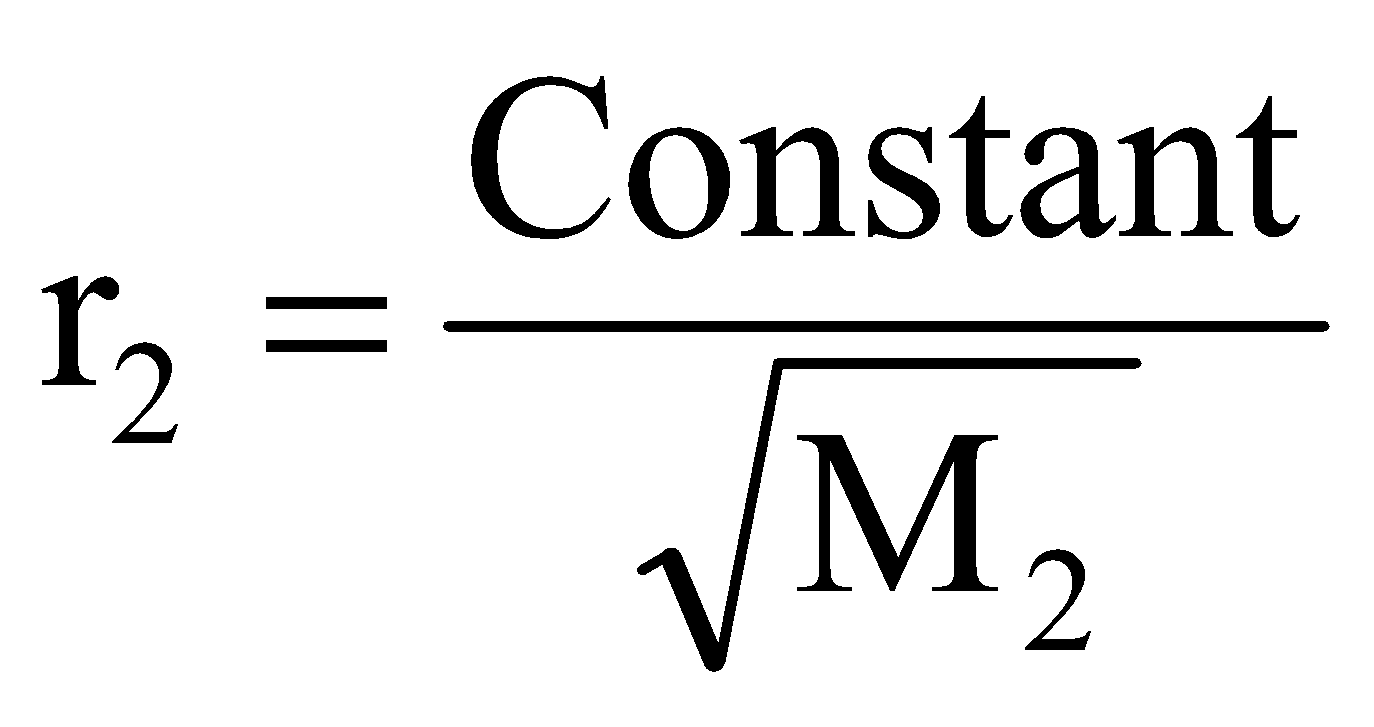 ..... (ii)
..... (ii)
Dividing (i) by (ii), we get,
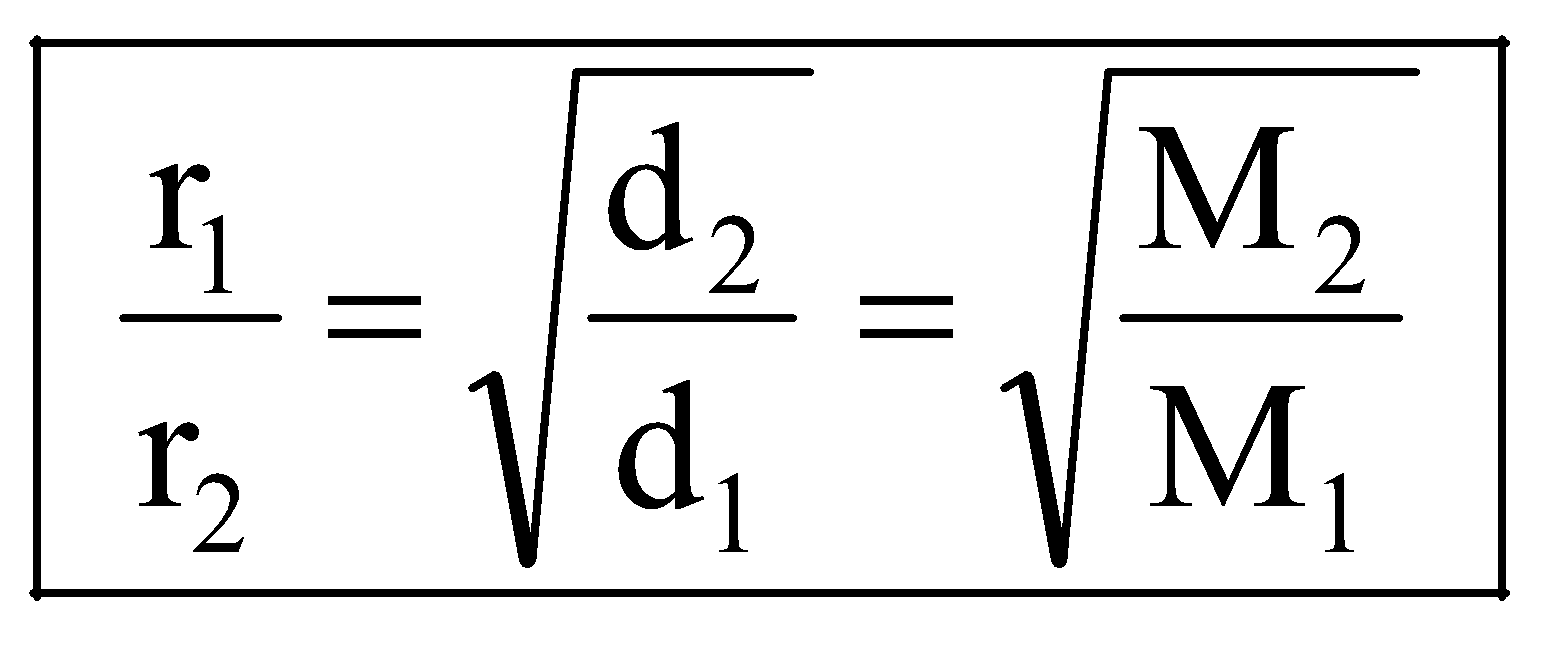
Example 7 720dm3 of SO2 diffuse through a porous partition in 60s. What volume of O2 will diffuse under similar conditions in 30s?
Solution: 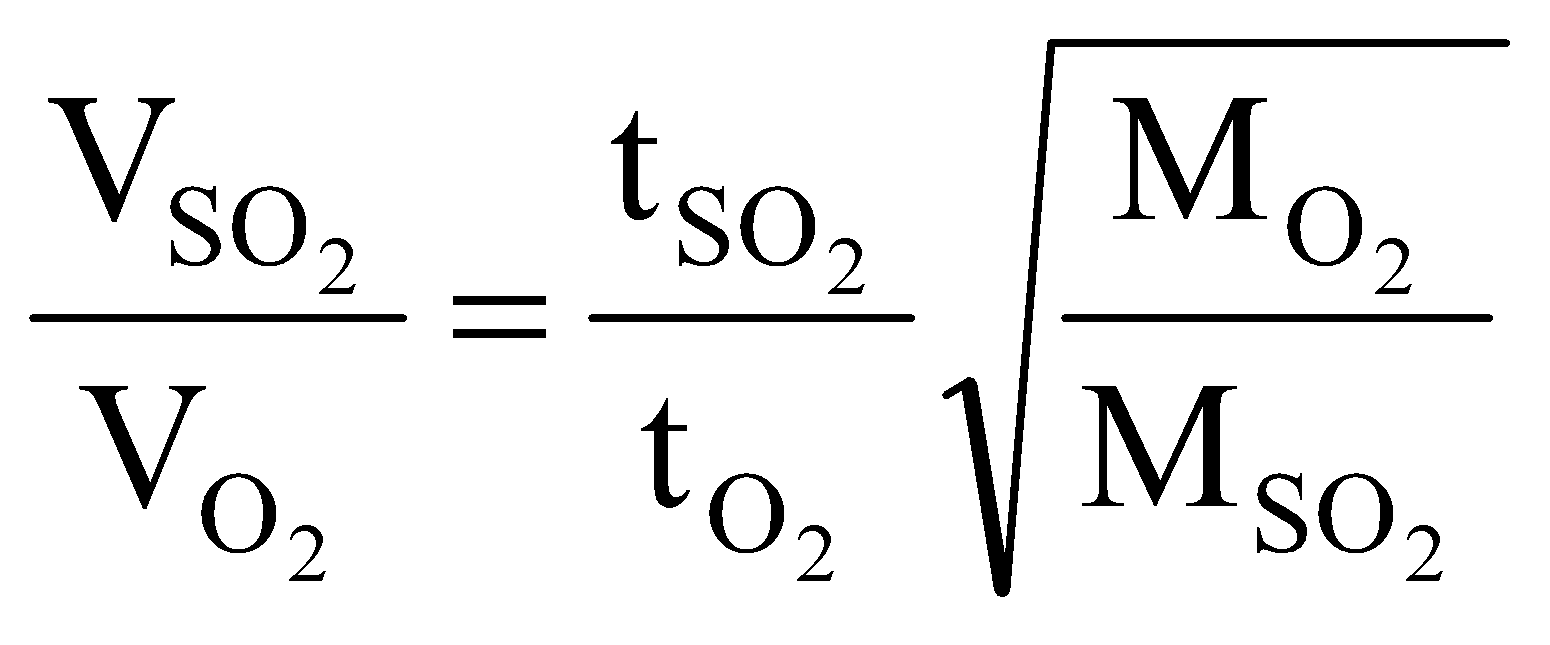
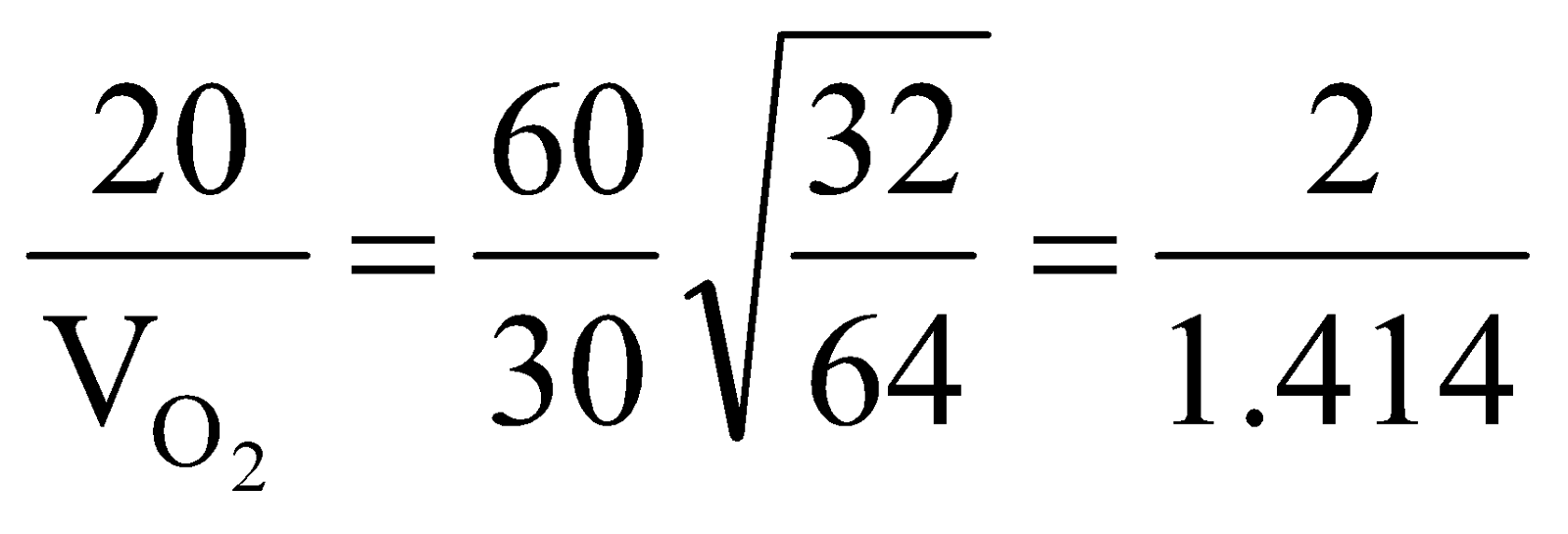
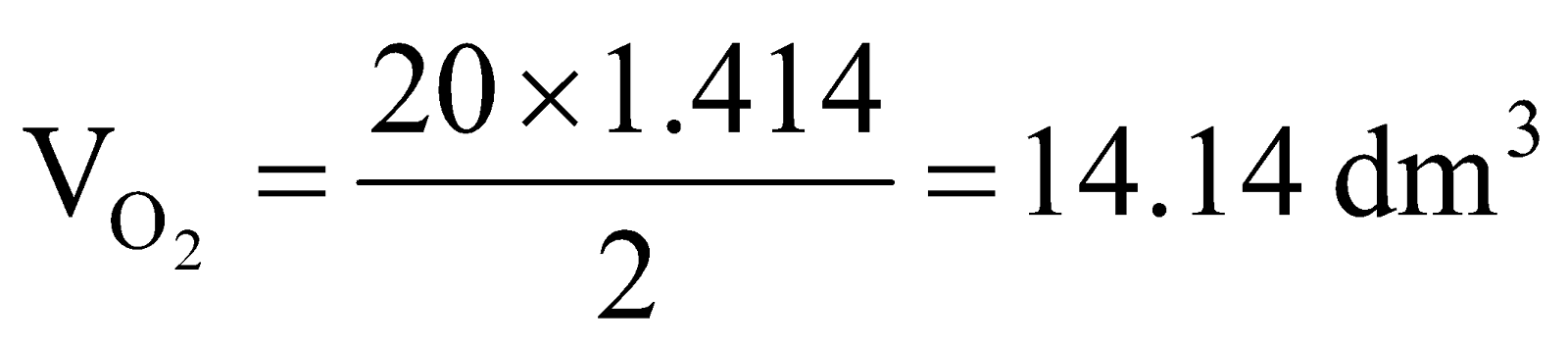 .
.
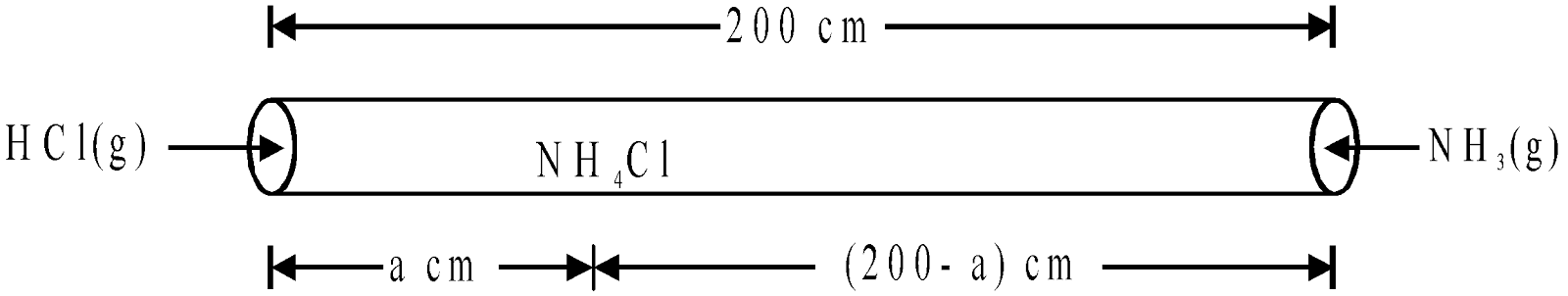
Example 8 Through the two ends of a glass tube of length 200cm HCl(g) and NH3(g) are allowed to enter. At what distance NH4Cl will first appear?
Solution: NH4Cl will appear first at a distance where HCl(g) and NH3(g) will meet. Let after time ‘t’ they meet at a distance of ‘a’ cm from HCl end as shown below,
We know, 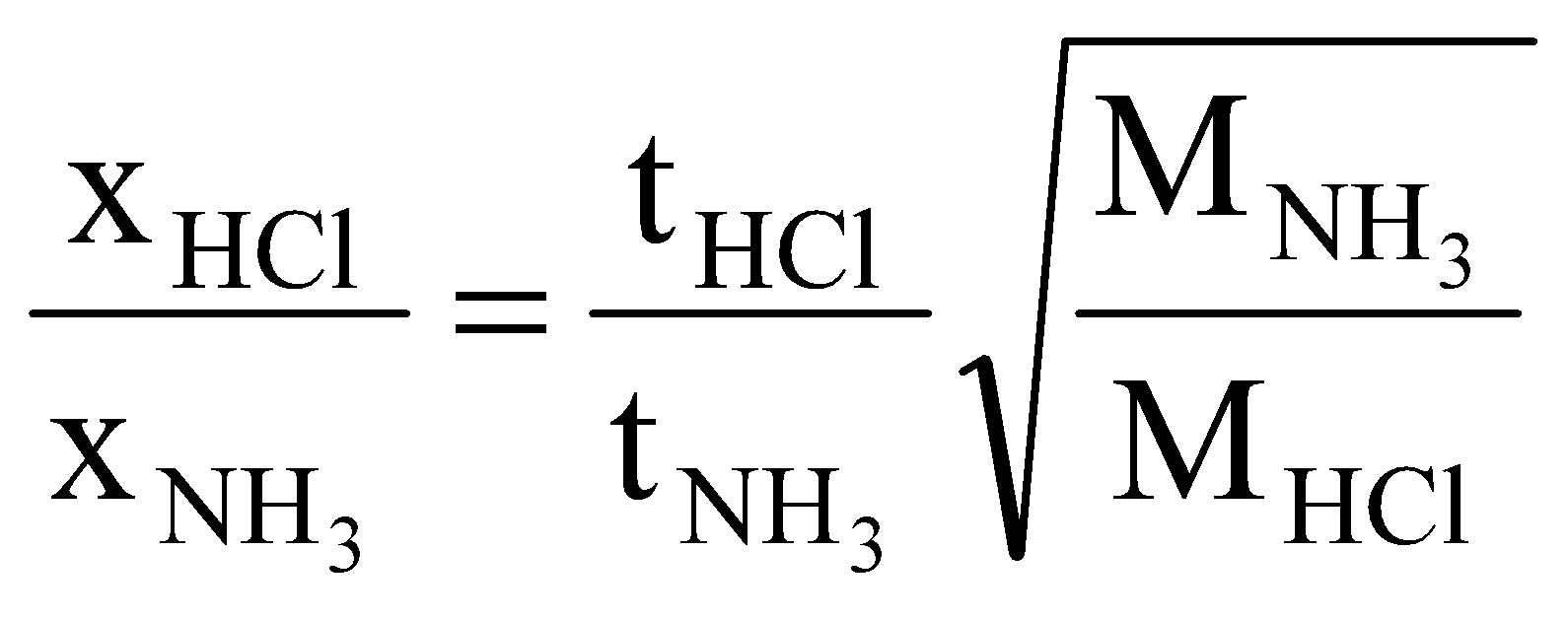
Here, 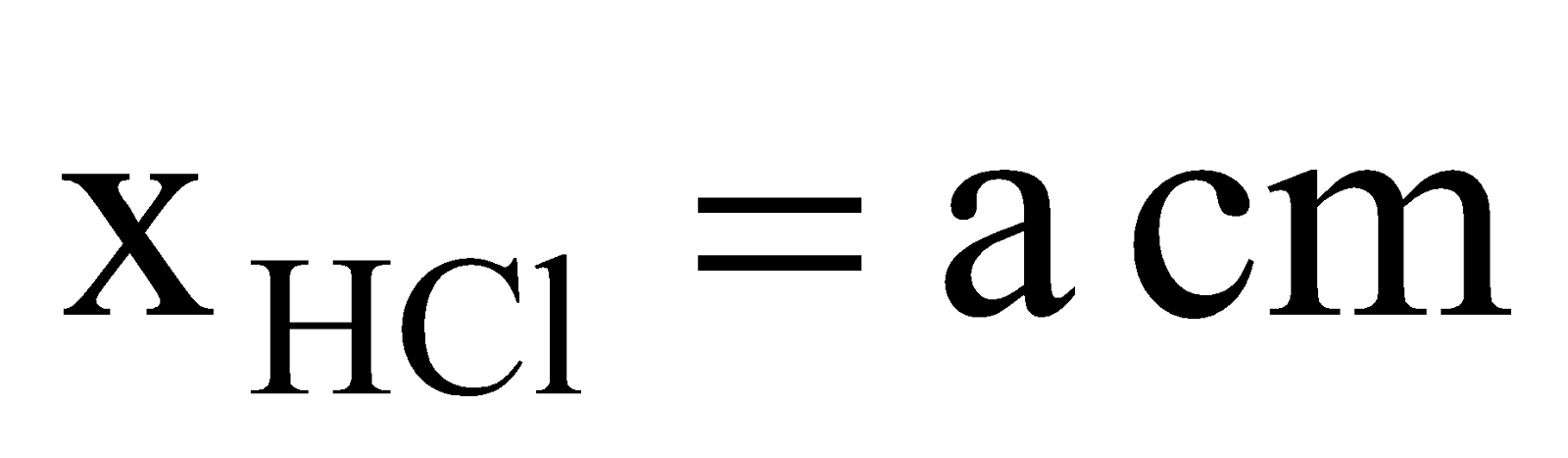 ,
, 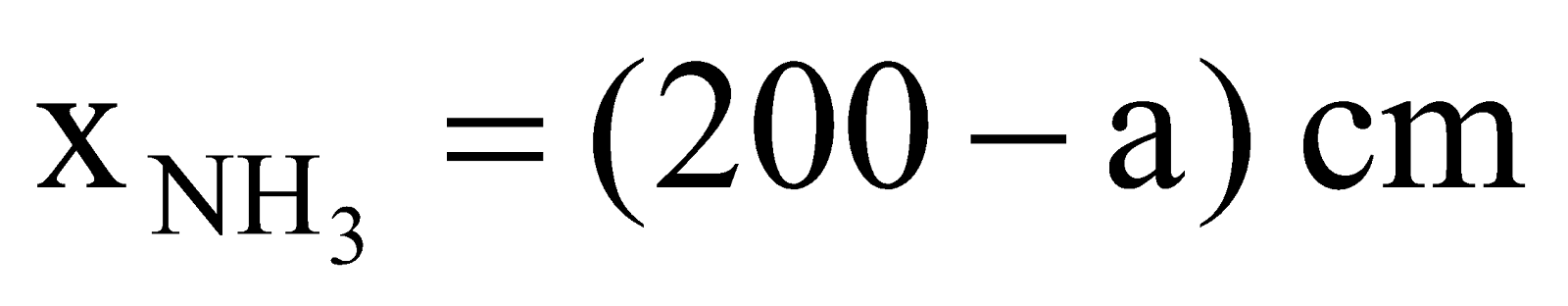 ,
, 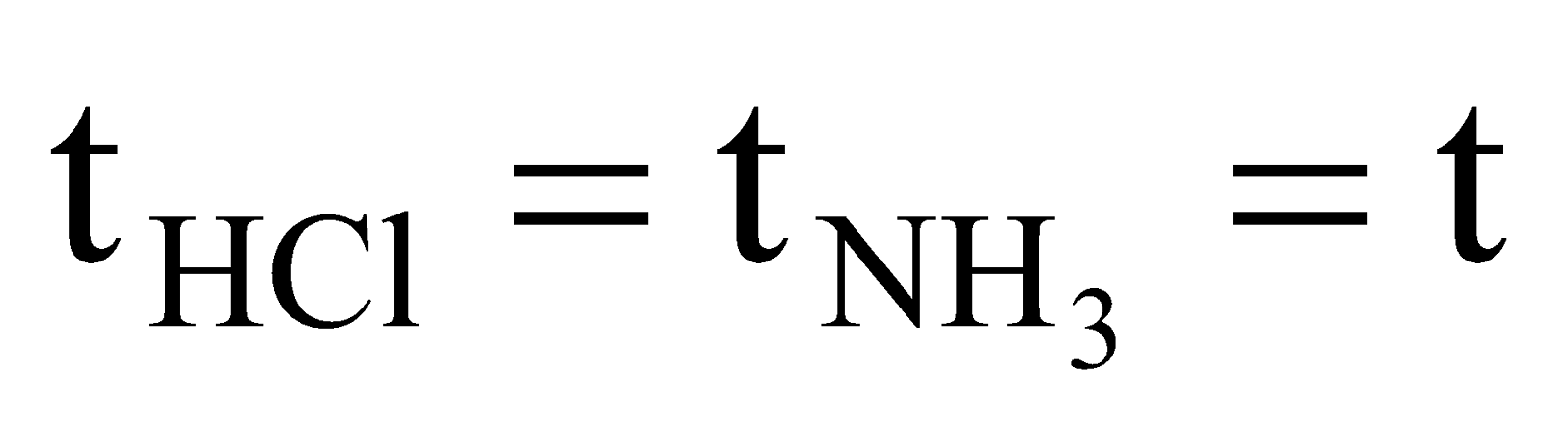 ,
, 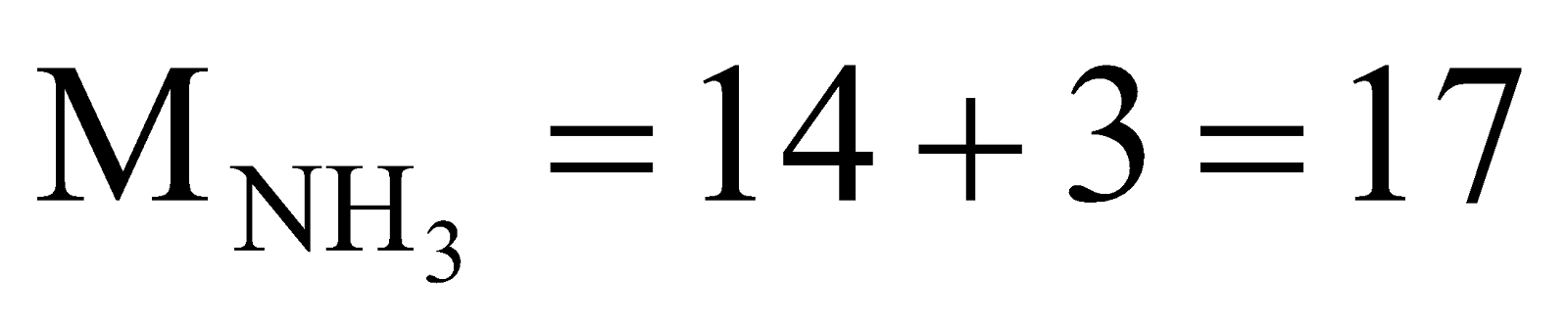 ,
,
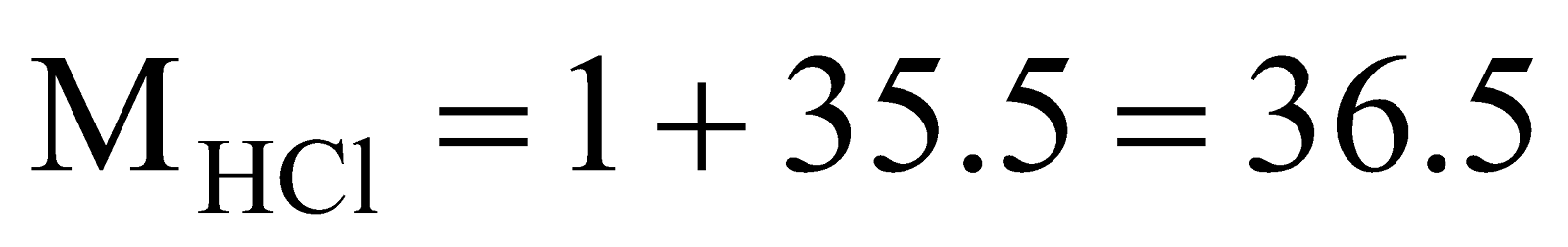 .
.
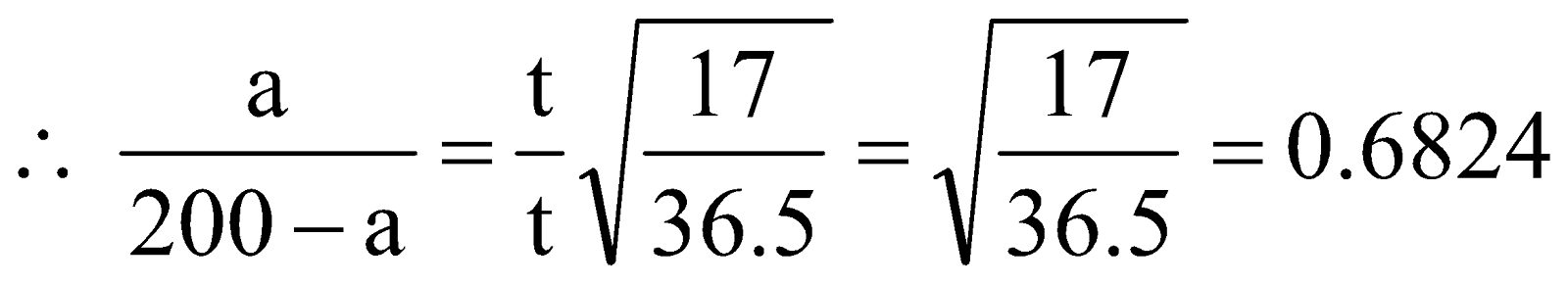
Solving for ‘a’ we get, 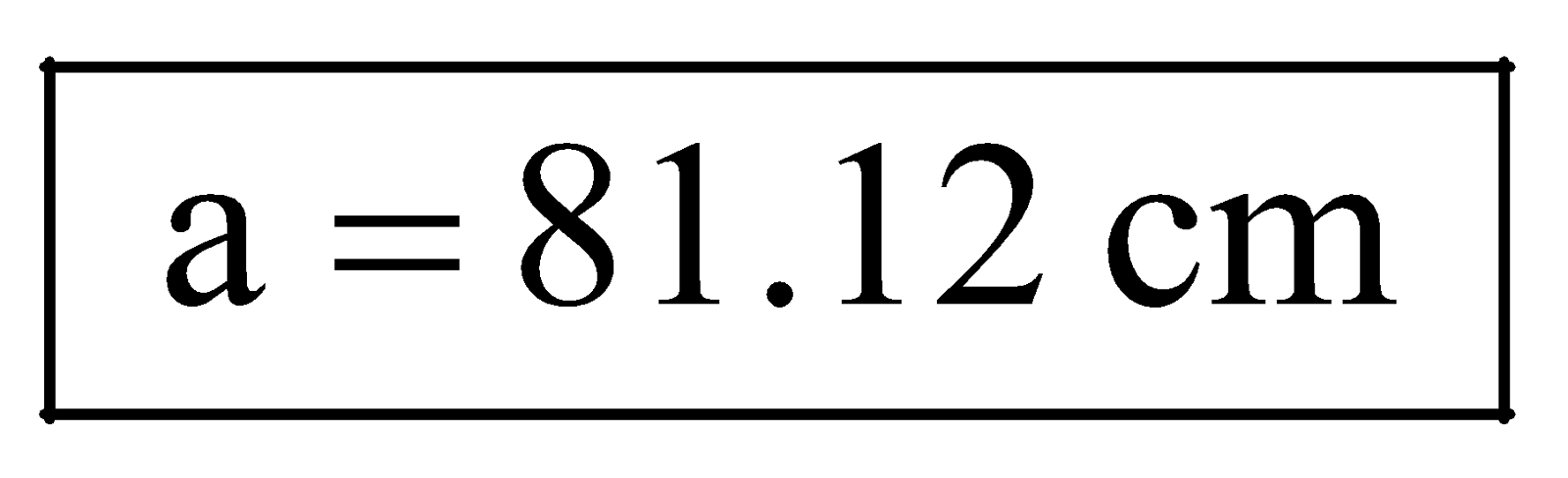 .
.
It means NH4Cl will first appear at a distance of 81.12 cm from HCl end.
Example9 Which of the two gases ammonia and hydrogen chloride will diffuse faster and by what factor?
Solution: 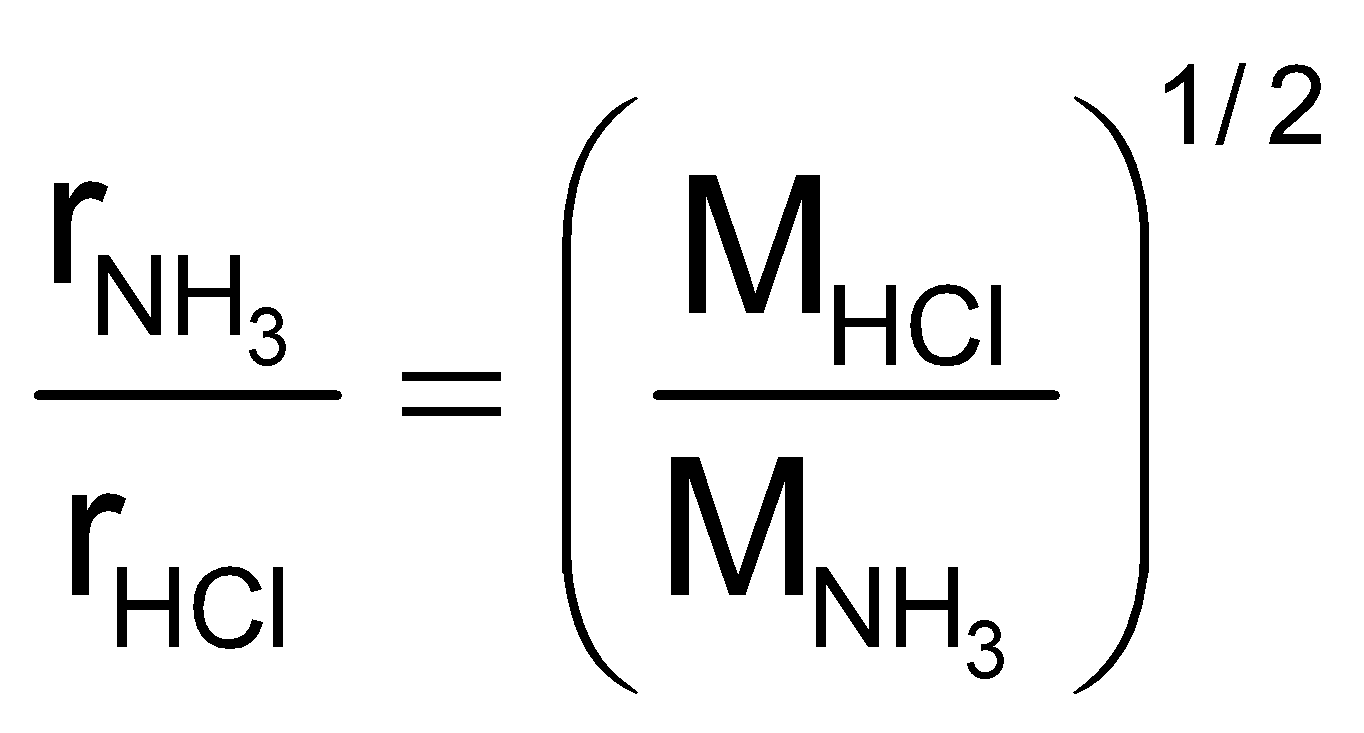
= 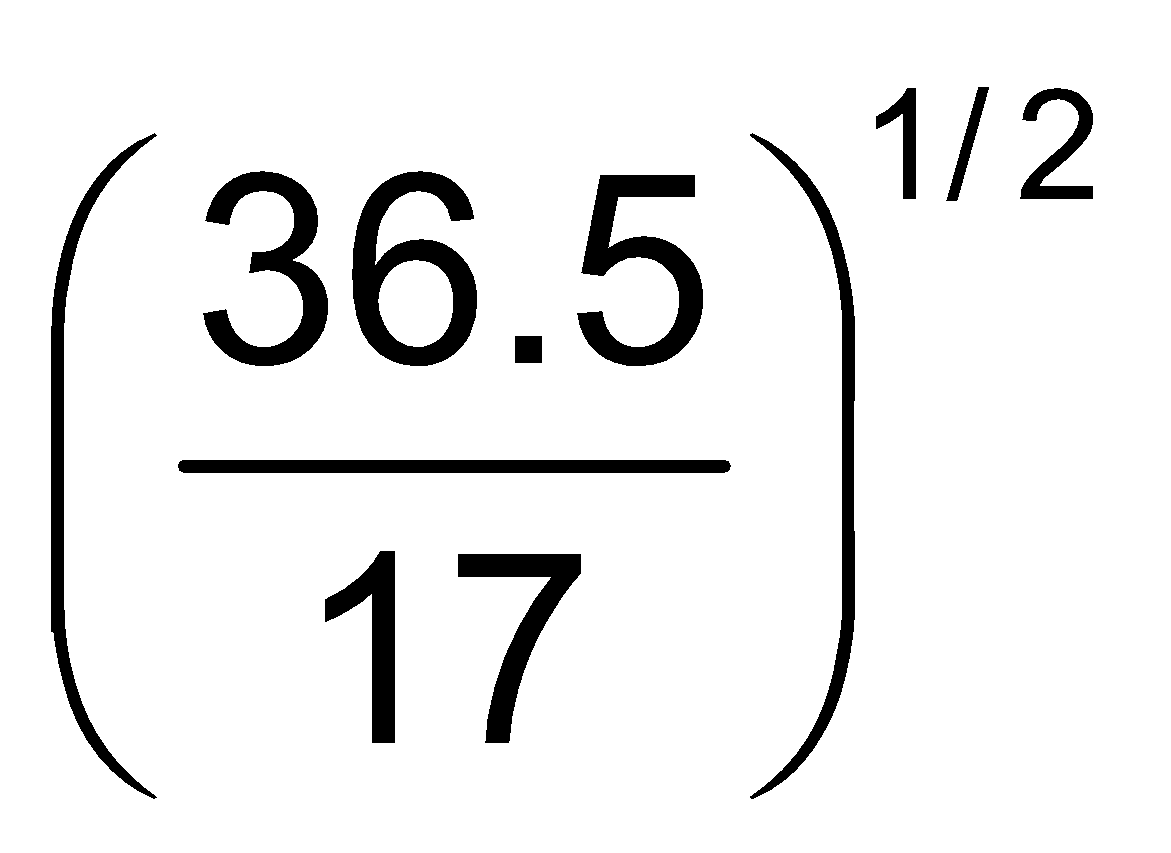 = 1.46 or
= 1.46 or 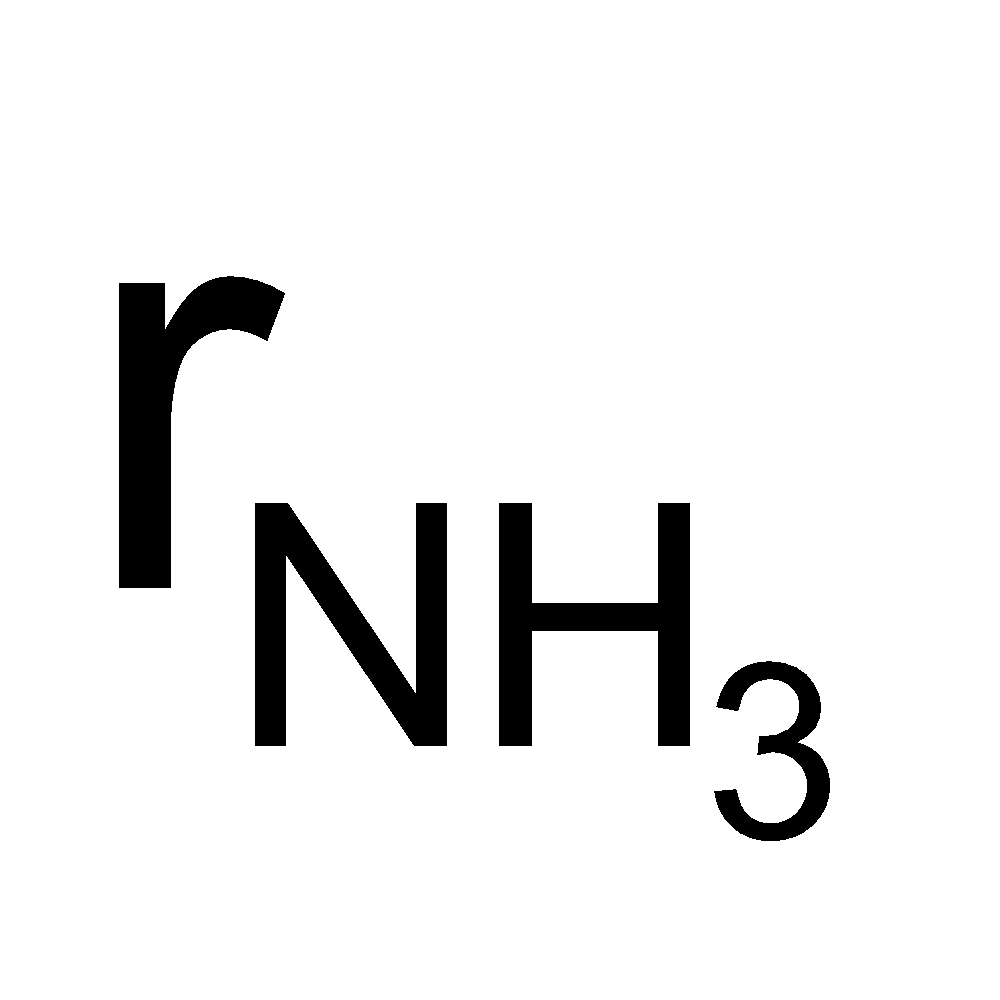 = 1.46 rHCl
= 1.46 rHCl
Thus ammonia will diffuse 1.46 times faster than hydrogen chloride gas










