KINETIC MOLECULAR THEORY OF GASES
States of matter of Class 9
KINETIC MOLECULAR THEORY OF GASES
The various gas laws which we have studied so far were based an experimental facts.
The theory that provides an explanation for the various experimental observations about a gas is based on the Kinetic Molecular model. It is assumed that all gases are made up of identical molecules, which are in a state of constant random motion.
Postulates of the Model
The following are the postulates of the model:
- A gas consists of a large number of identical molecules of mass m. The dimensions of these molecules are very small compared to the space between them. Hence the molecules are treated as point masses.
- There are practically no attractive forces between the molecules. The molecules therefore more independently.
- The molecules are in a state of ceaseless and random motion, colliding with each other and with the walls of the container. The direction of their motions changes only on collision. These collisions are known as elastic collisions in which the energy and momenta of the molecules are concerned. In non−elastic collisions these quantities are not conserved.
- The pressure of a gas is the result of collision of molecules with the wall of the container.
- The average kinetic energy of the colliding molecules is directly proportional to its temperature.
Velocities of gas molecules
Average Velocity
As per kinetic theory of gases, each molecule is moving with altogether different velocity. Let ‘n’ molecules be present in a given mass of gas, each one moving with velocity v1, v2, v3… vn. The average velocity or UaV = average of all such velocity terms.
Average velocity = 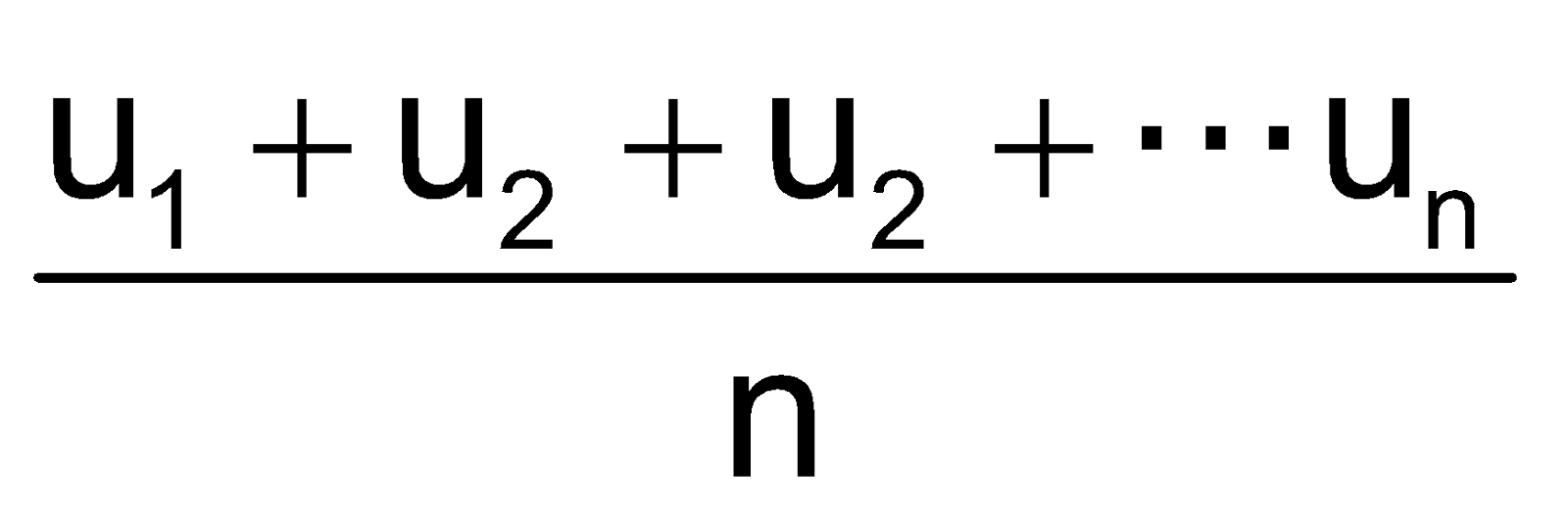 =
= 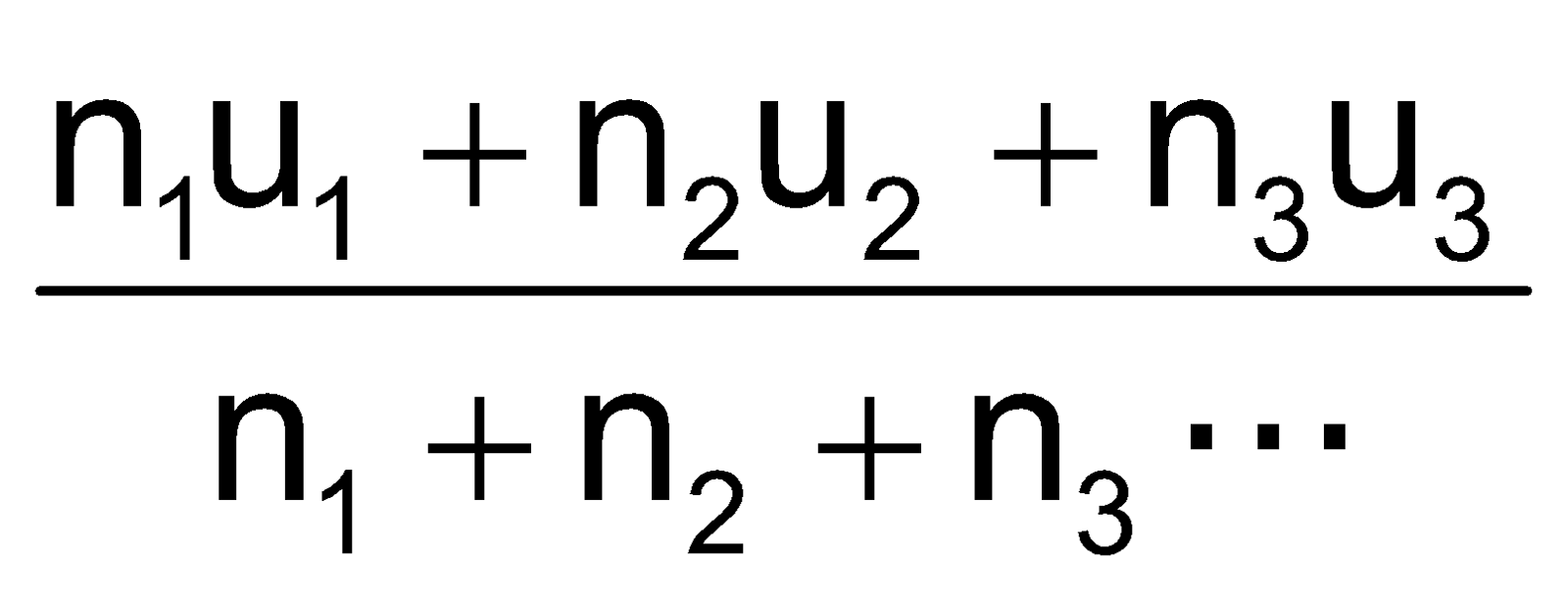
Uav = 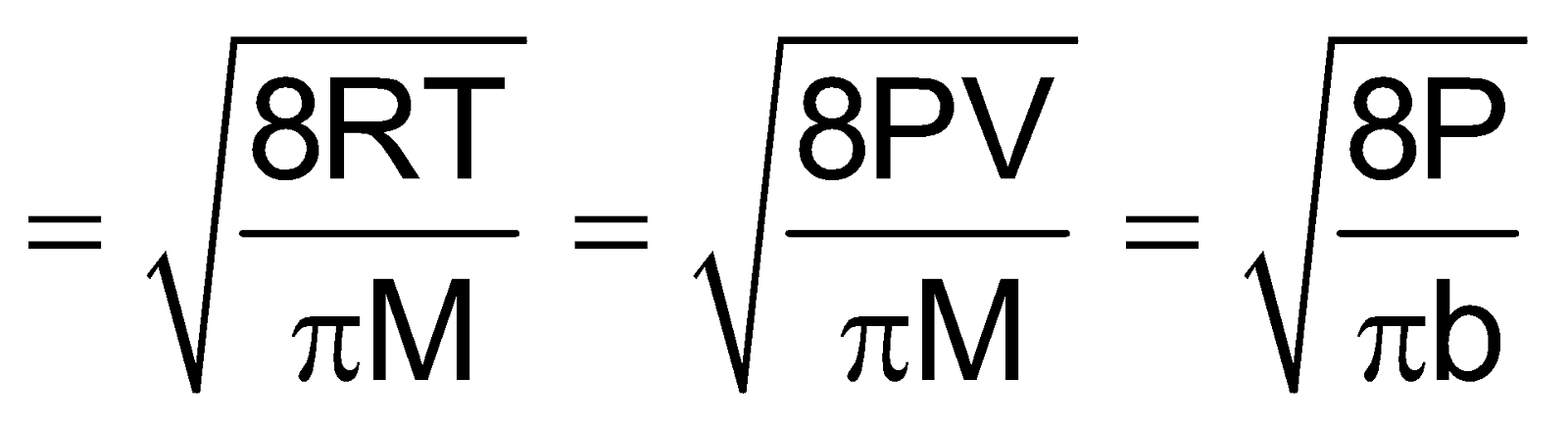
Root Mean Square Velocity
Maxwell proposed the term Urms as the square root of means of square of all such velocities.
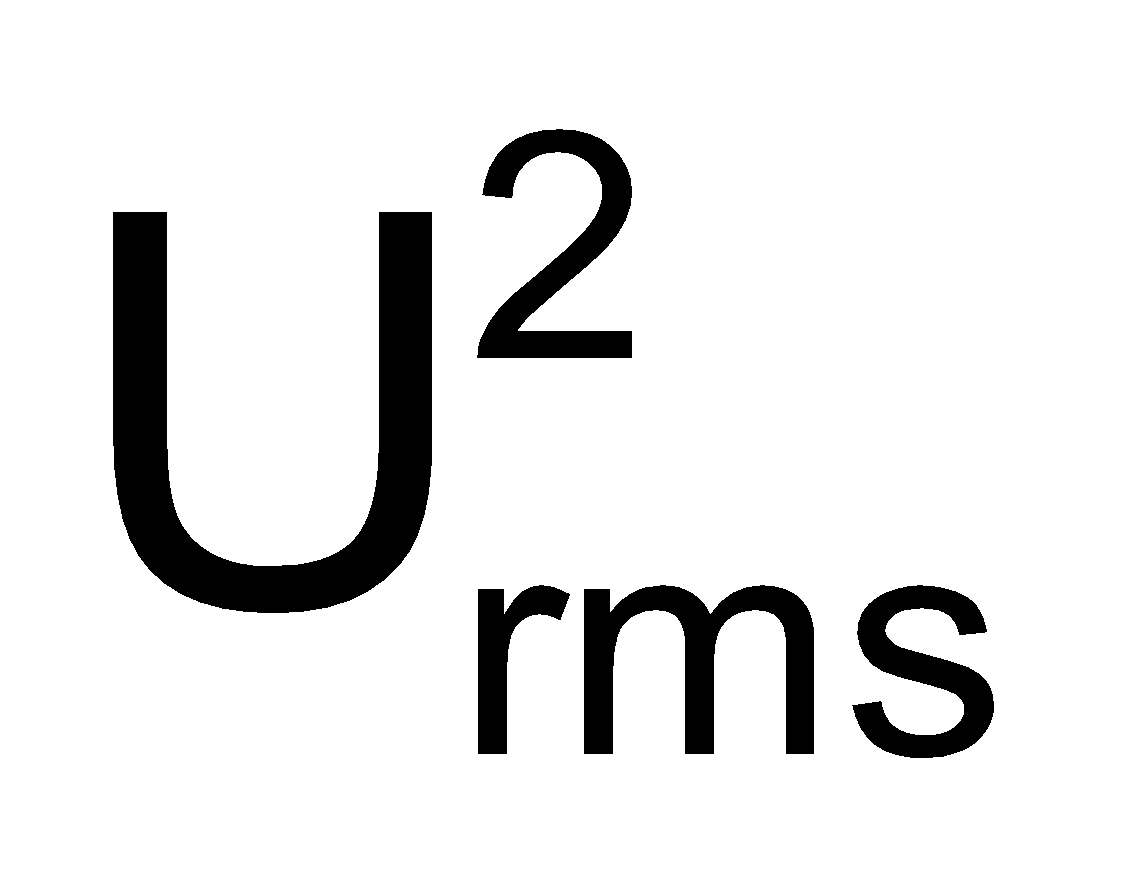 =
= 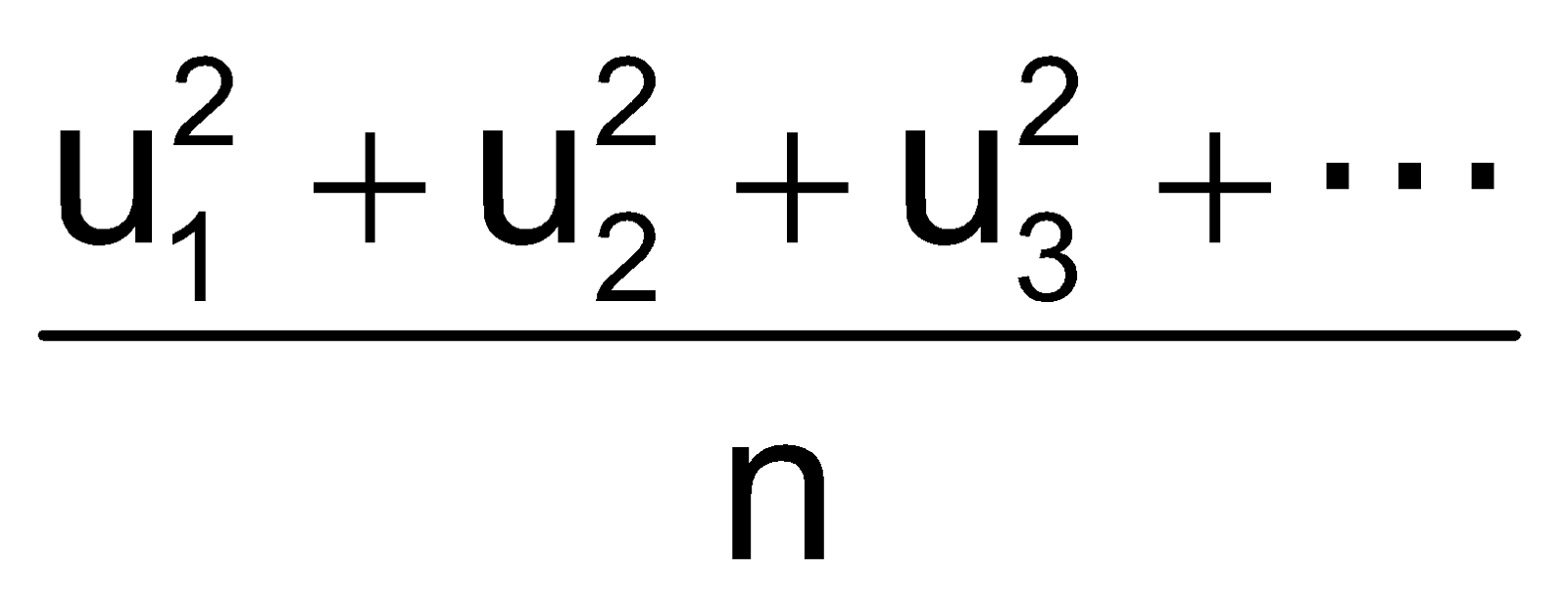 =
=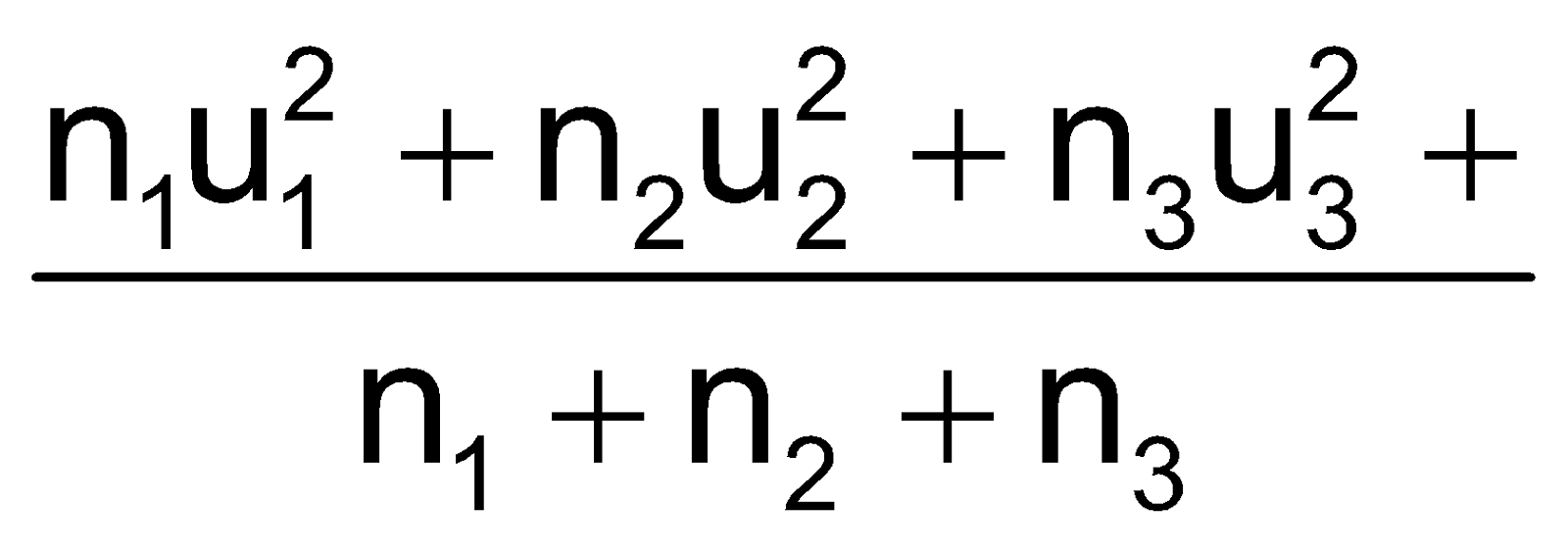
Also Urms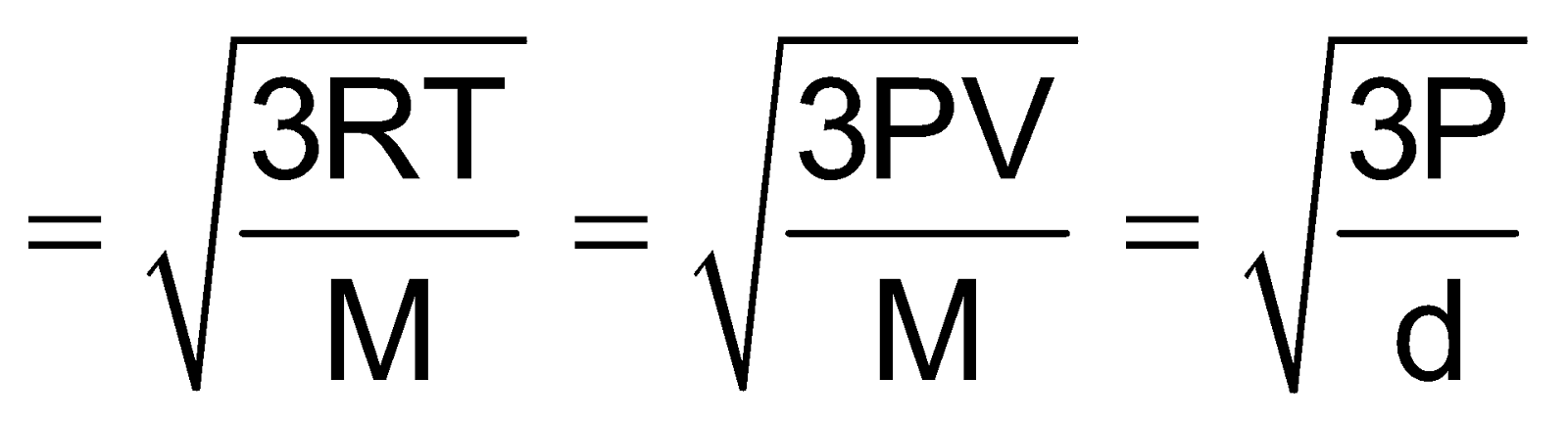
Most probable velocity
It is the velocity which is possessed by maximum no. of molecules.
Vmp= 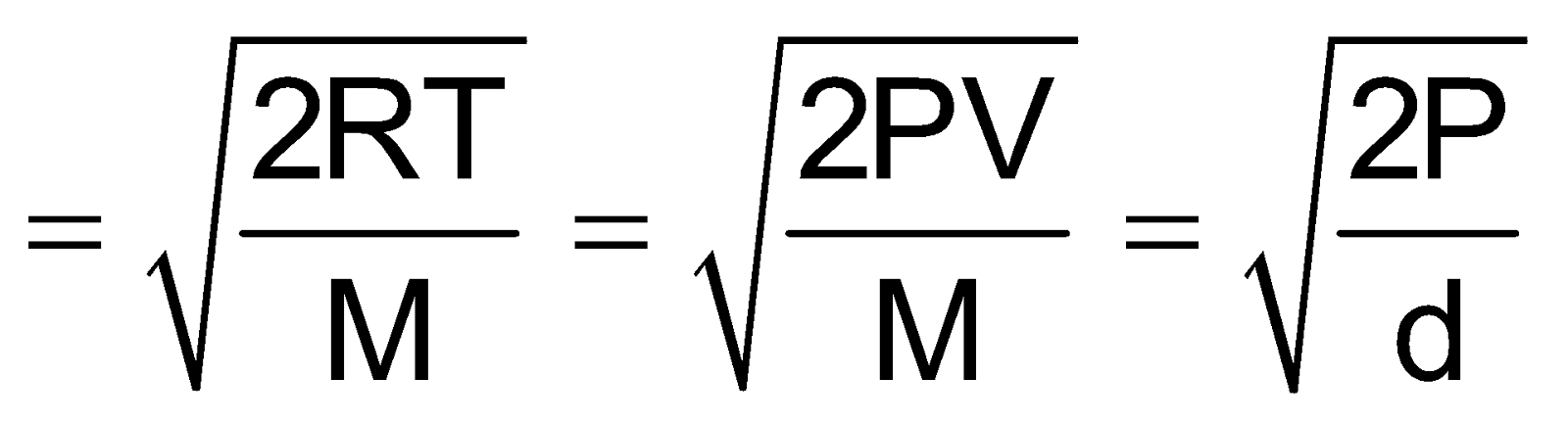
Furthermore UMP : UAV : Urms : :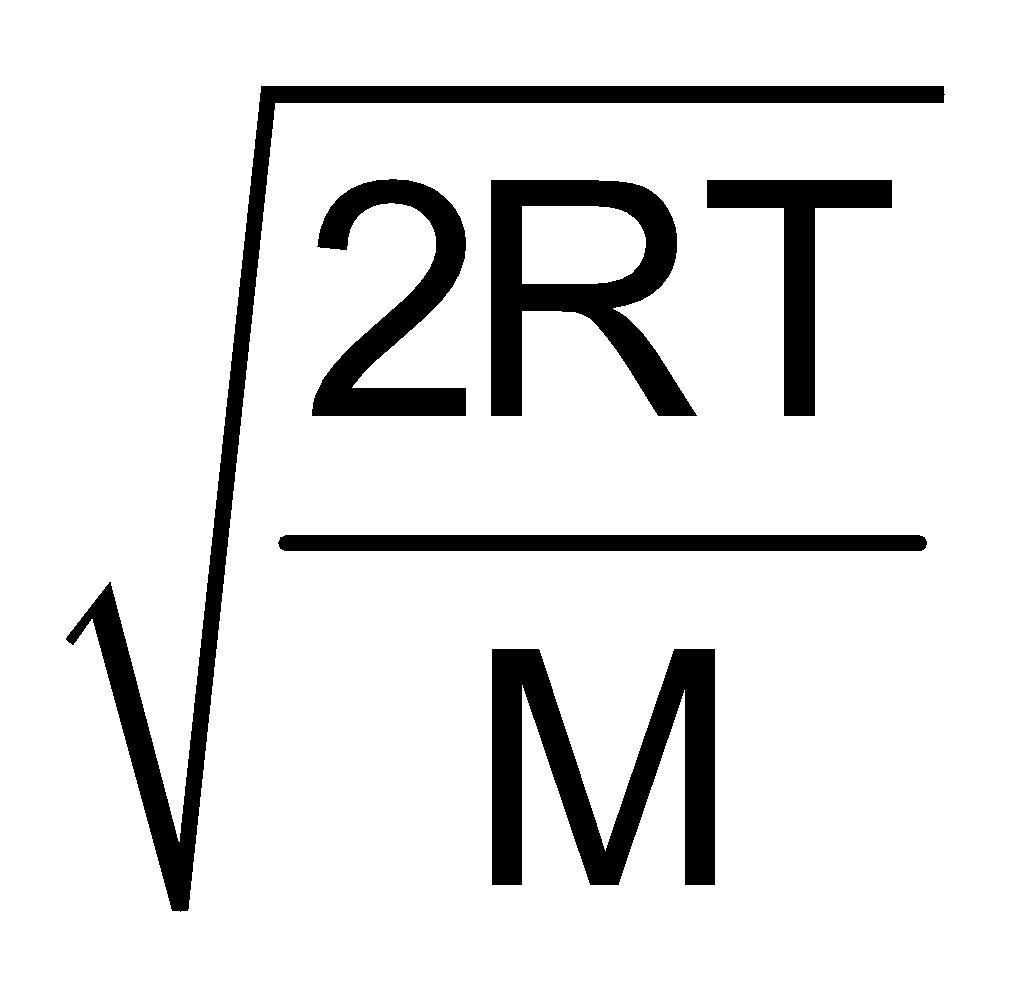 :
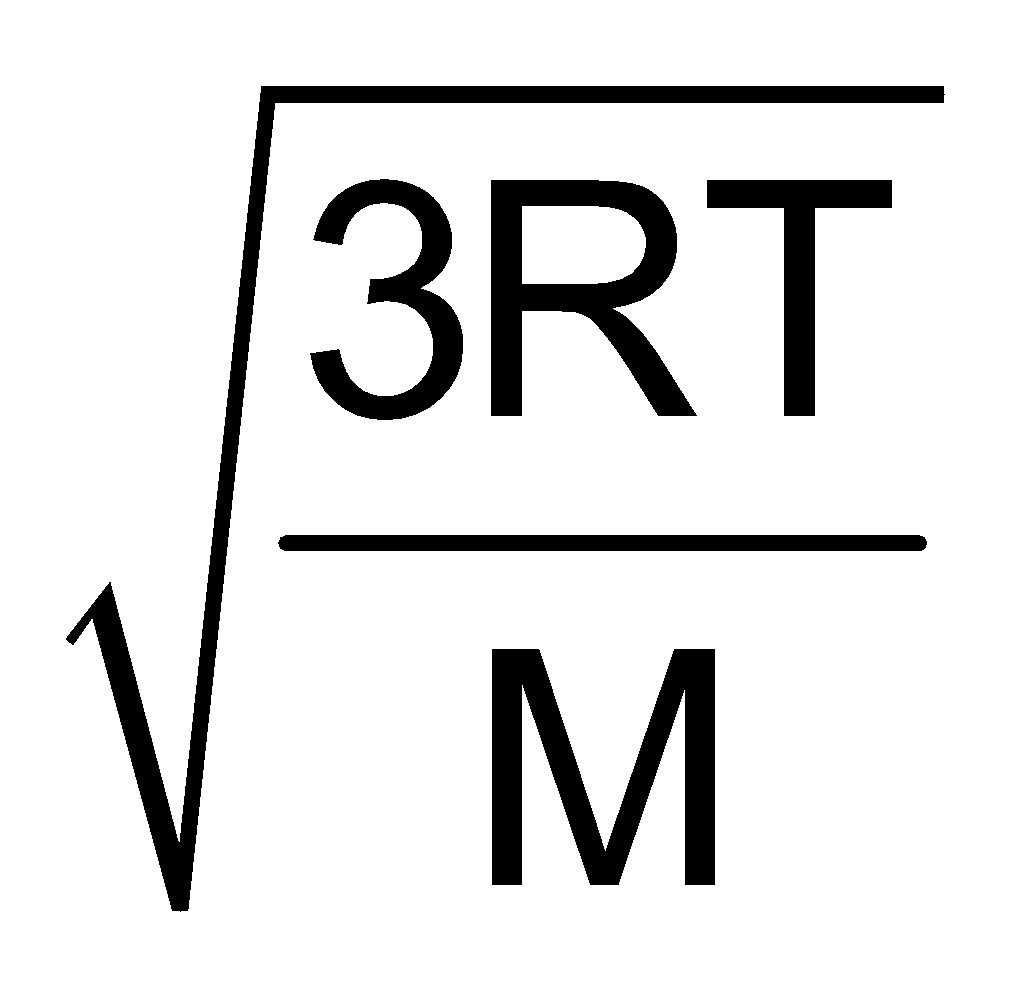
: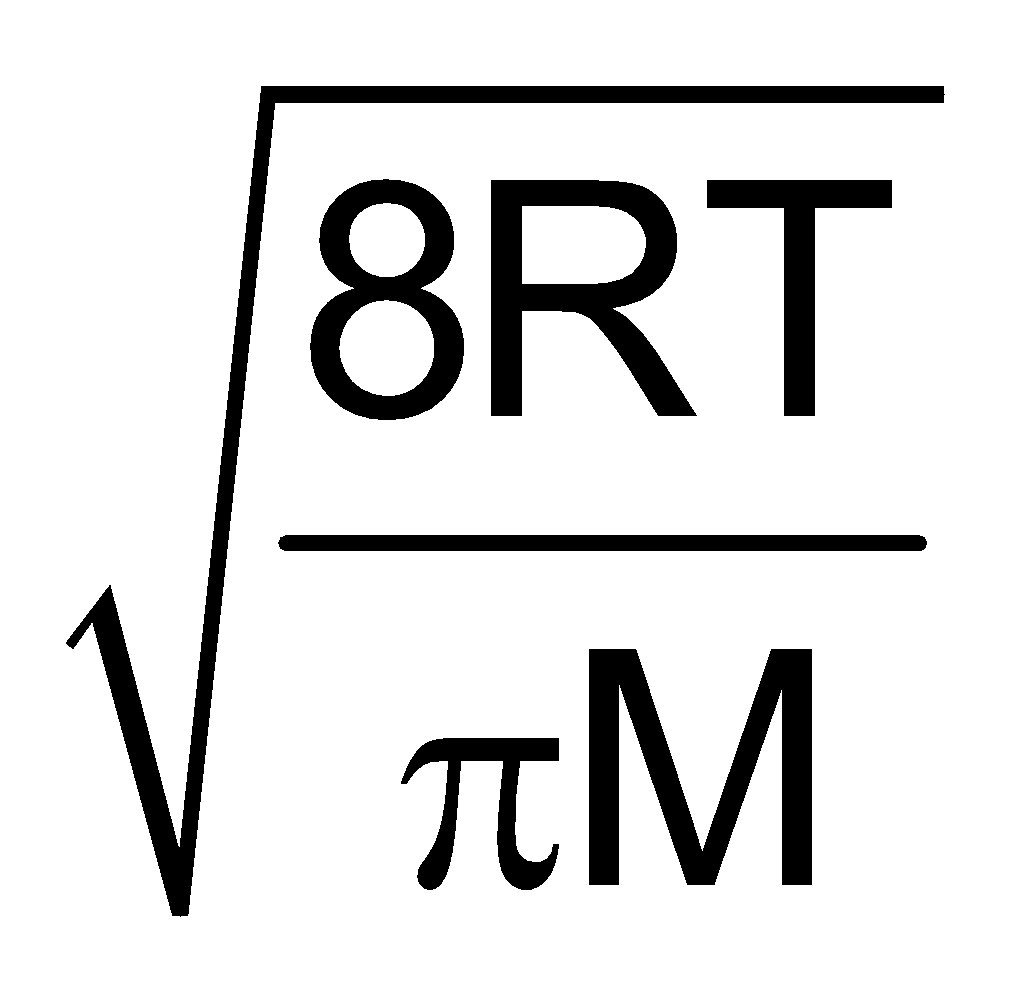 :
: 
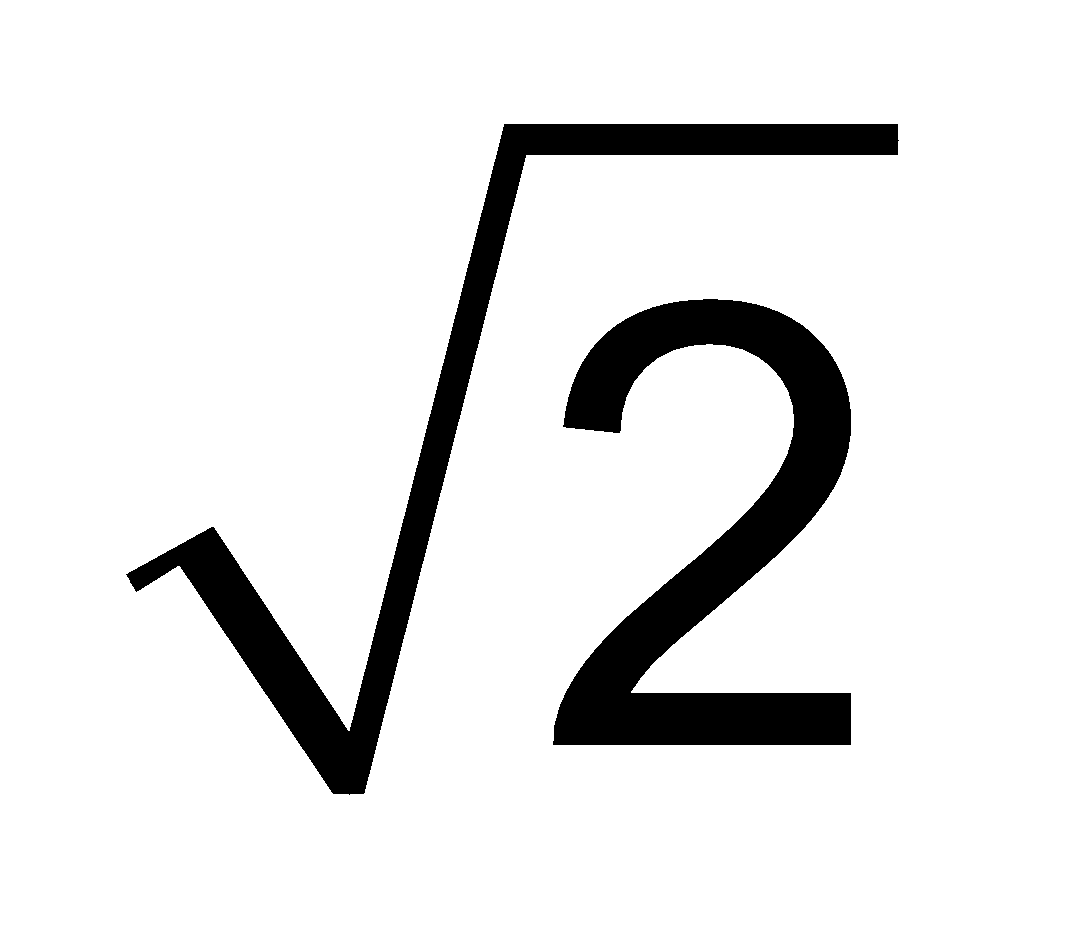 :
:  :
:
1 : 1.128 : 1.224
Also Uav = Urms× 0.9213
Example 10 What is r.m.s. speed and most probable speed of O2 as at 27ºC?
Solution: 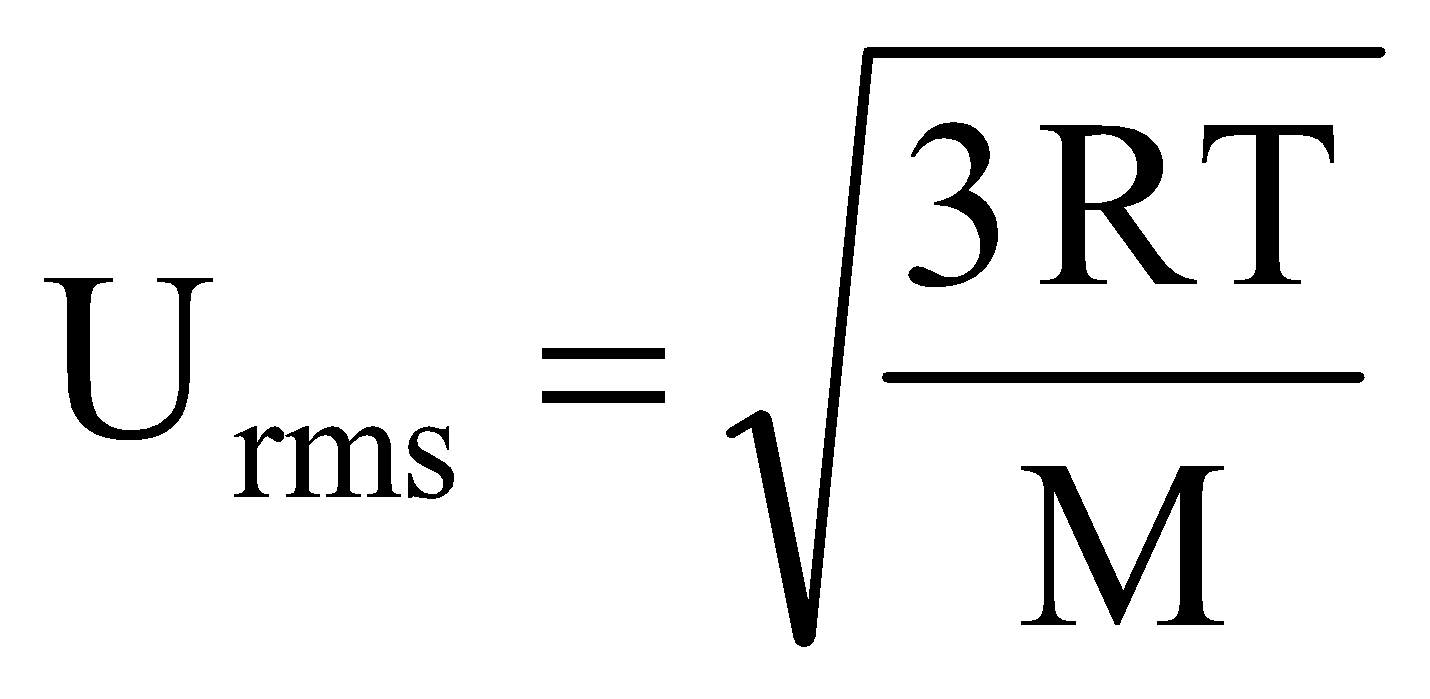
Take, R in 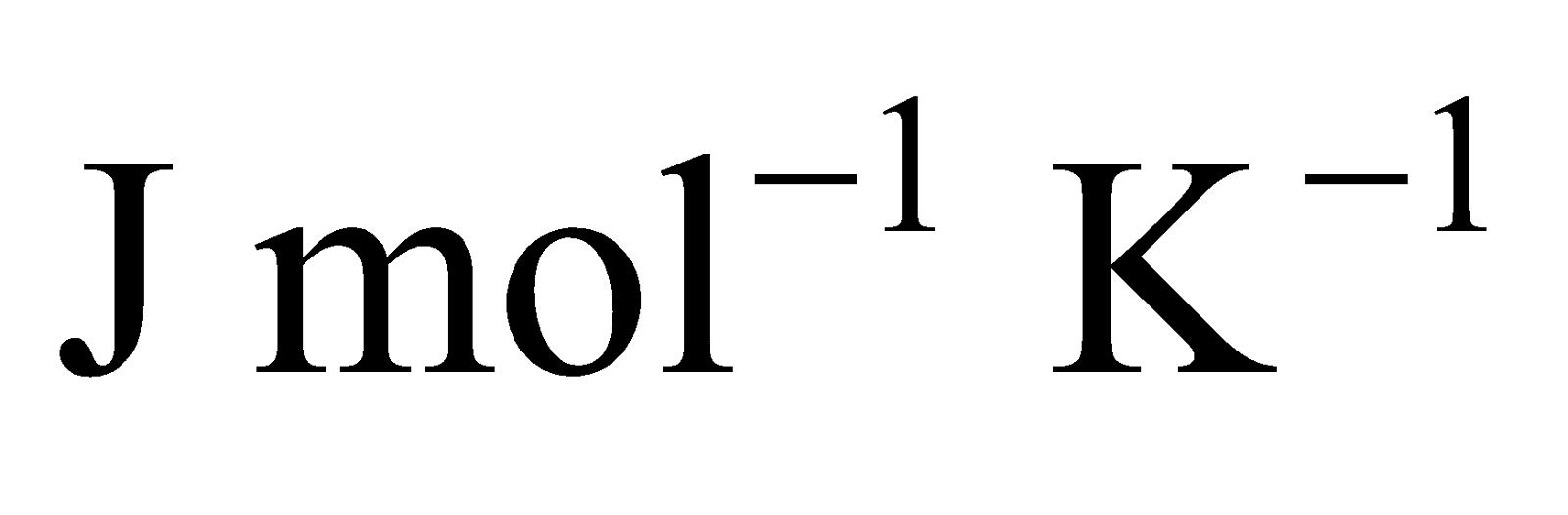 and M in kg to have speed in m/s.
and M in kg to have speed in m/s.
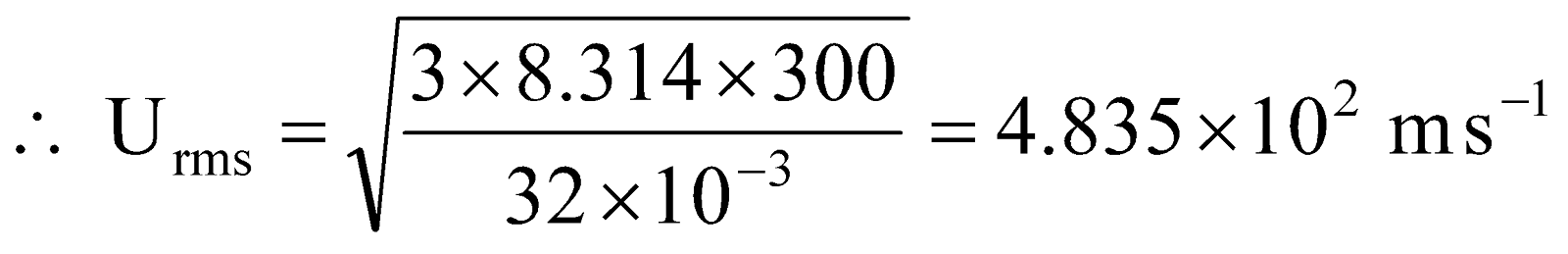 .
.
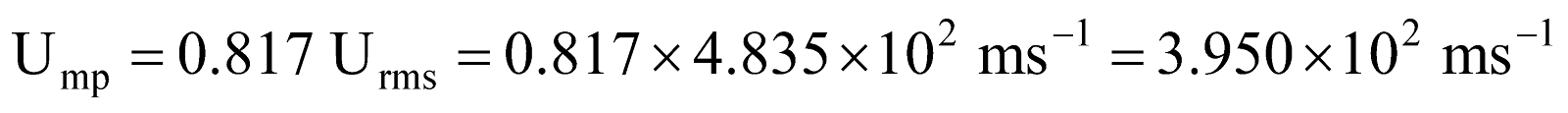 .
.