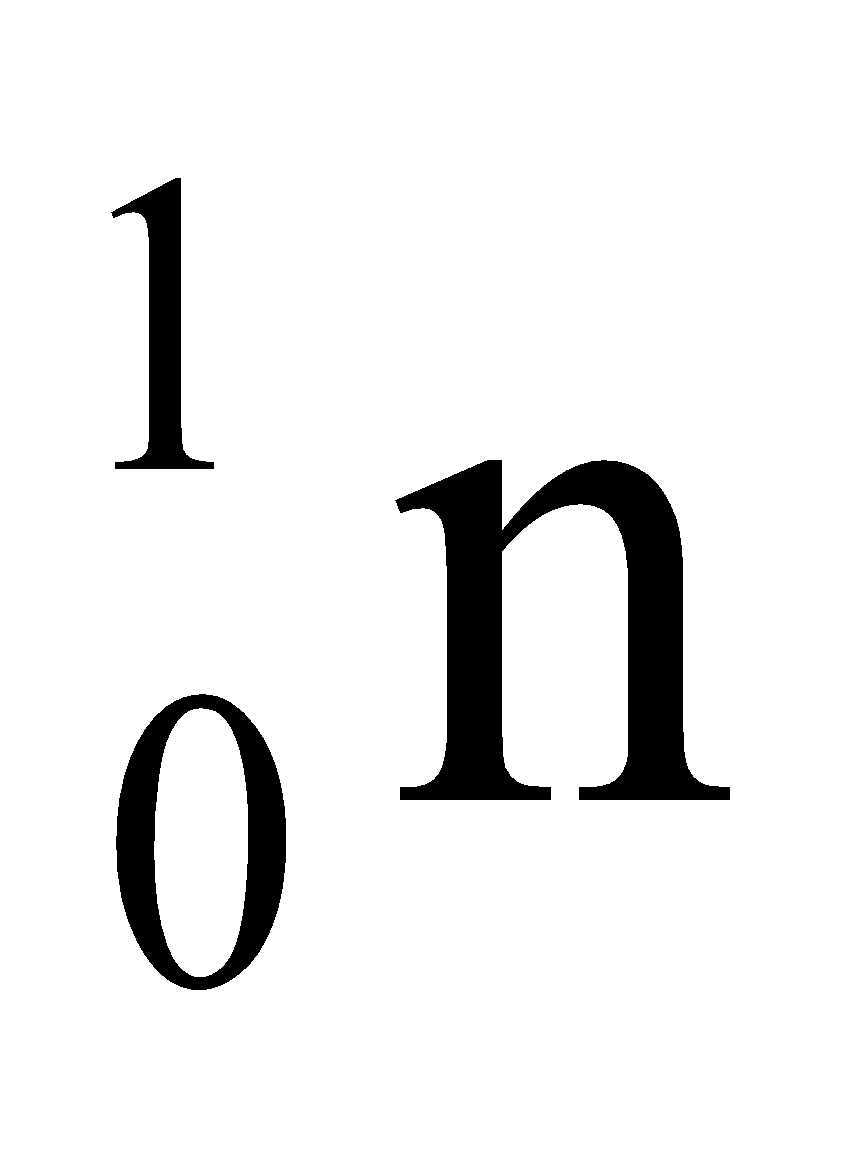FUNDAMENTAL PARTICLES OF AN ATOM
Structure of atom of Class 9
FUNDAMENTAL PARTICLES OF AN ATOM
Electron:
The name electron was proposed by Stoney and was discover in cathode ray experiments. Electron has a negative charge on it, its mass is 1/1837 times the mass of one atom of hydrogen. It is denoted by the symbol 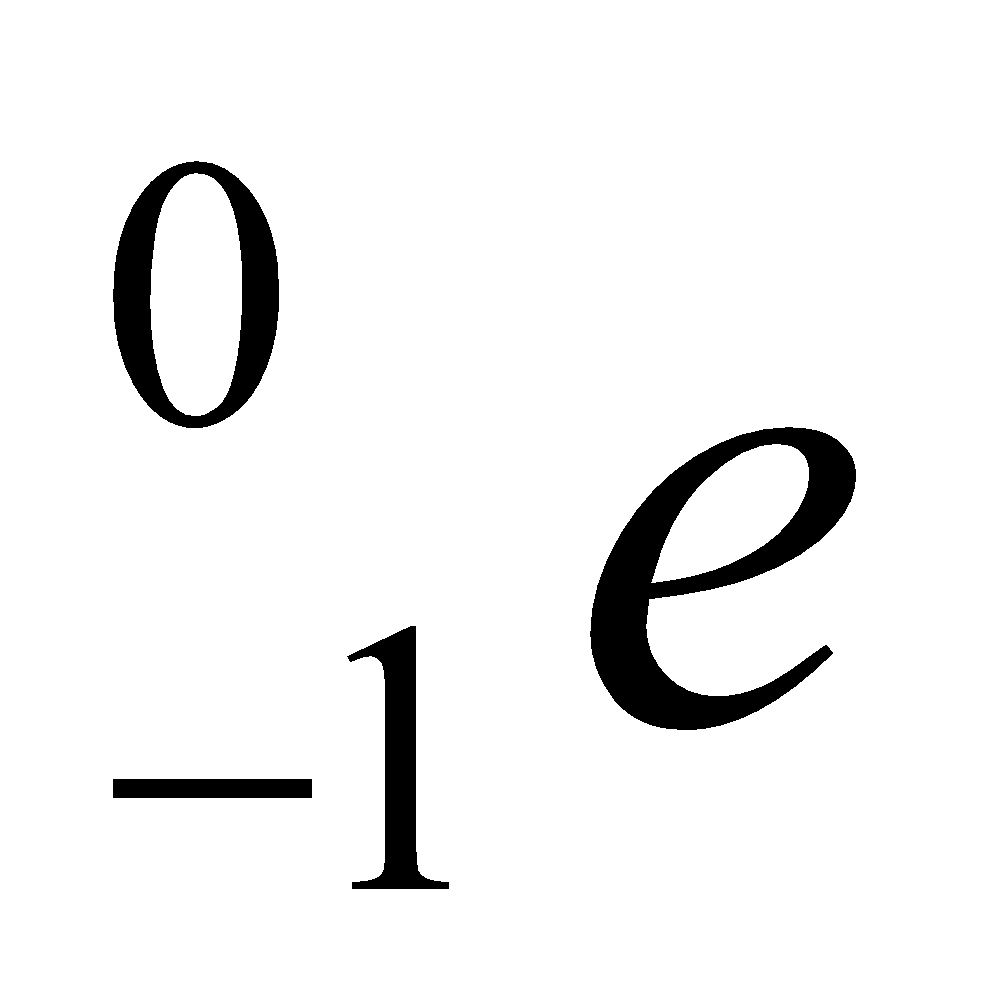 , where 0 denotes its mass and -1 denotes its charge. Electrons in the outer most shell are called valence electrons.(Mass of one mol electron = 0.55 gm)
, where 0 denotes its mass and -1 denotes its charge. Electrons in the outer most shell are called valence electrons.(Mass of one mol electron = 0.55 gm)
Proton:
Proton was discovered in anode ray experiment by E.Goldstein. Proton has a unit positive charge, it is denoted by the symbol 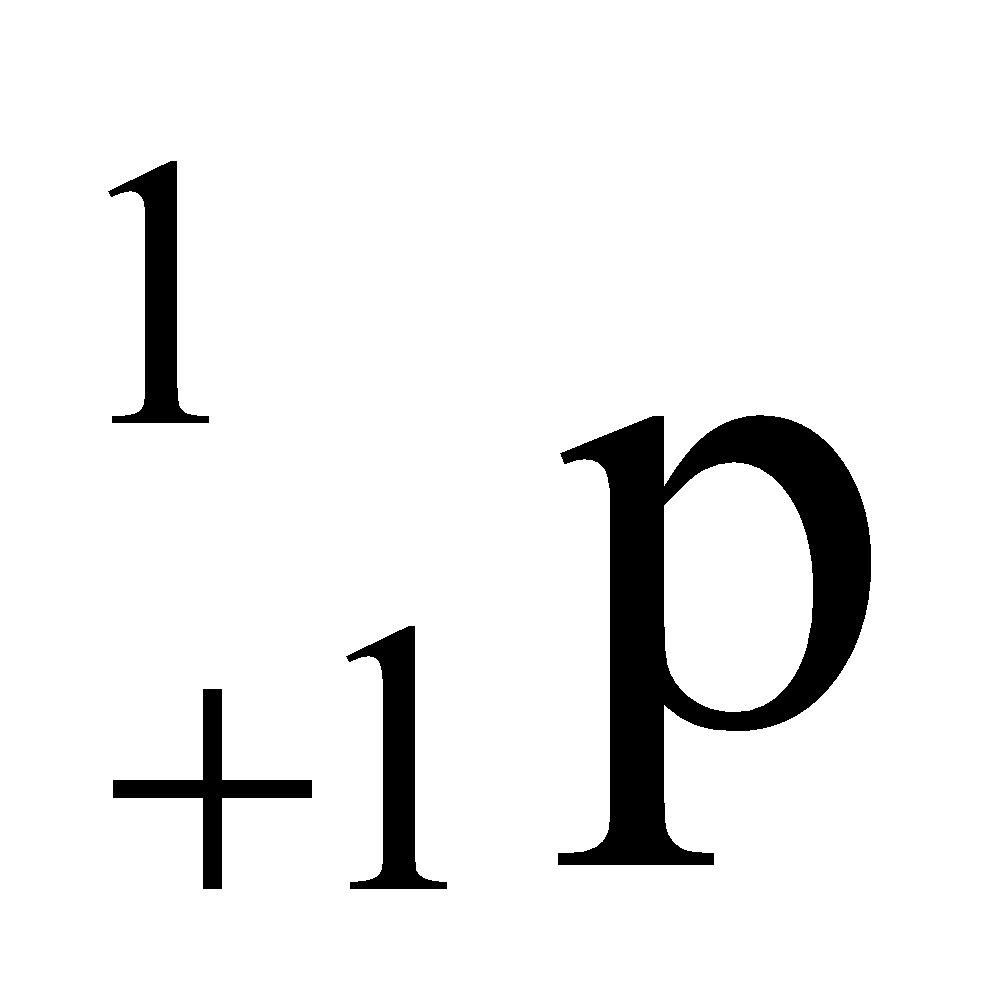 , where 1 denotes it atomic mass and +1 denotes its charge. .(Mass of one mole proton = 1.007 gm)
, where 1 denotes it atomic mass and +1 denotes its charge. .(Mass of one mole proton = 1.007 gm)
Neutron:
Neutron has no electric charge on it. Its mass is almost equal to the mass of one atom of hydrogen. It is denoted by the symbol 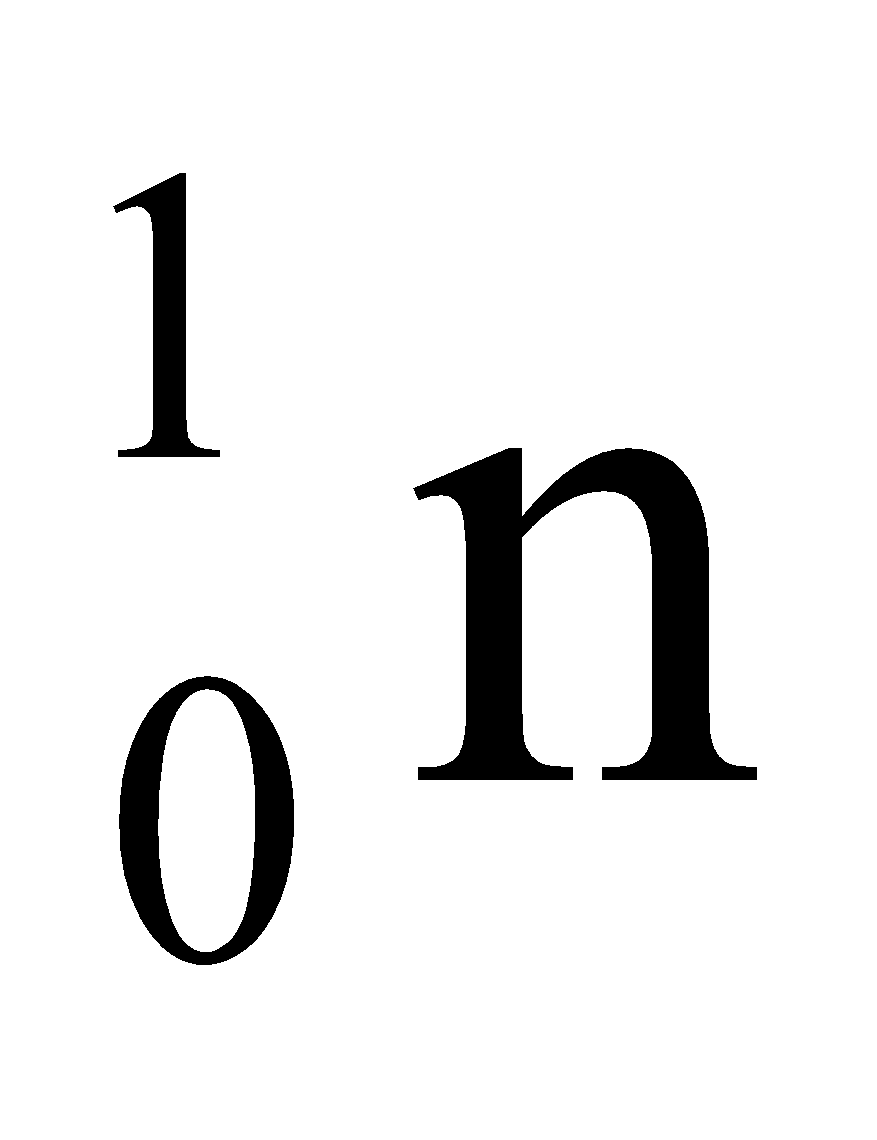 , where 1 denotes its atomic mass and 0 denotes its charge.
, where 1 denotes its atomic mass and 0 denotes its charge.
|
Property |
Electron |
Proton |
Neutron |
|
1. Discovery |
J.J. Thomson |
E. Goldstein |
James Chadwick |
|
2. Symbol |
e |
p |
n |
|
3. Nature |
Negatively charged |
Positively charged |
Neutral |
|
4. Relative charge |
–1 |
+1 |
0 |
|
5. Absolute charge |
1.602 × 10-19 C |
1.602 × 10-19 C |
0 |
|
6. Relative mass |
|
1 |
1 |
|
7. Absolute mass |
9.109 × 10-28 g |
1.6725 × 10-24 g |
1.6748 × 10-24 g |
DISCOVERY OF ELECTRON:
The existence of electrons in an atom was shown by J.J. Thomson in 1897. He passed electricity at high voltage through a gas at very low pressure taken in a discharge tube. Let us discuss these studies which eventually led to the discovery of electrons.
What is a discharge tube?
A discharge tube is a long glass tube closed at both ends. Two circular metal plates A and B are sealed at the two ends of the tube as shown in figure. These circular plates are called electrodes. A side tube S is fused to the tube which can be connected to a vacuum pump (to suck out the air or gas present inside the tube to reduce the pressure inside the tube).
The two plates A and B are connected to a source of electricity to supply high voltage. The plate A connected to the negative terminal is cathode, whereas, the plate B connected to the positive terminal is called anode.
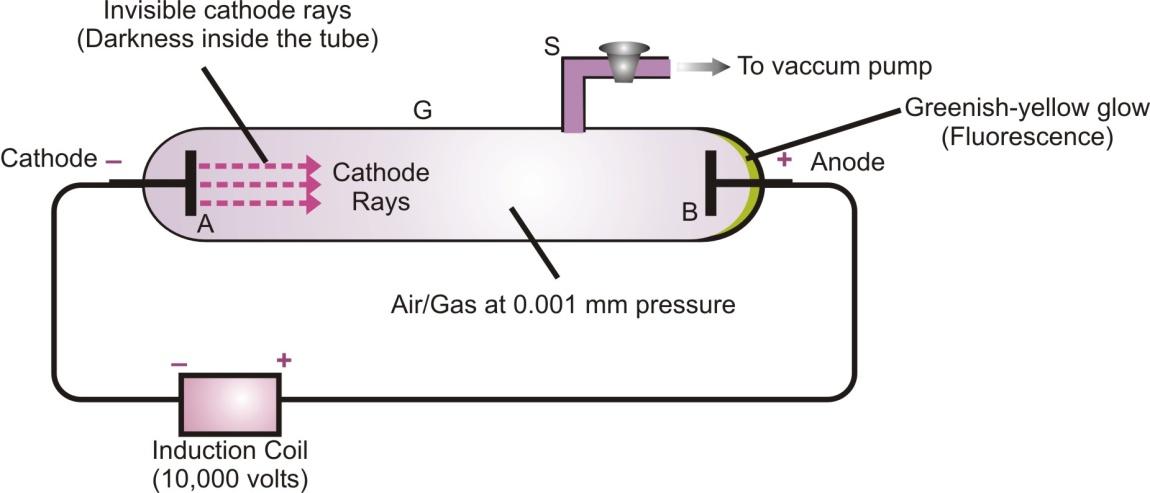
Production of cathode rays in a discharge tube
Properties of cathode rays
Cathode rays travel in straight lines. That is why, cathode rays cast shadow of any solid object placed in their path. The path cathode rays travel is not affected by the position of the anode.
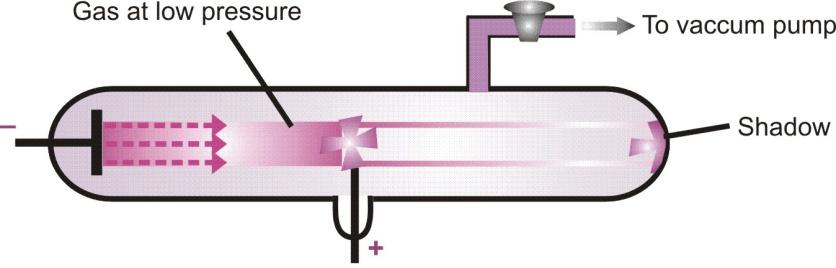
Production of shadow of the object placed in the path of cathode rays.
Cathode rays consist of matter particles, and posses energy by the virtue of its mass and velocity. Cathode rays set a paddle wheel into motion when it is placed in the path of these rays one the bladder of the paddle wheel.
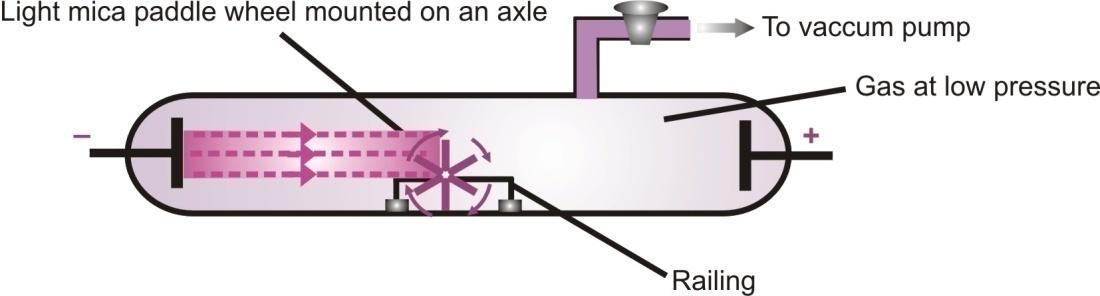
Rotation of light paddle
Cathode rays consist of negatively charged particles. When cathode rays are subjected to an electrical field, these get deflected towards the positively charge plate(Anode).
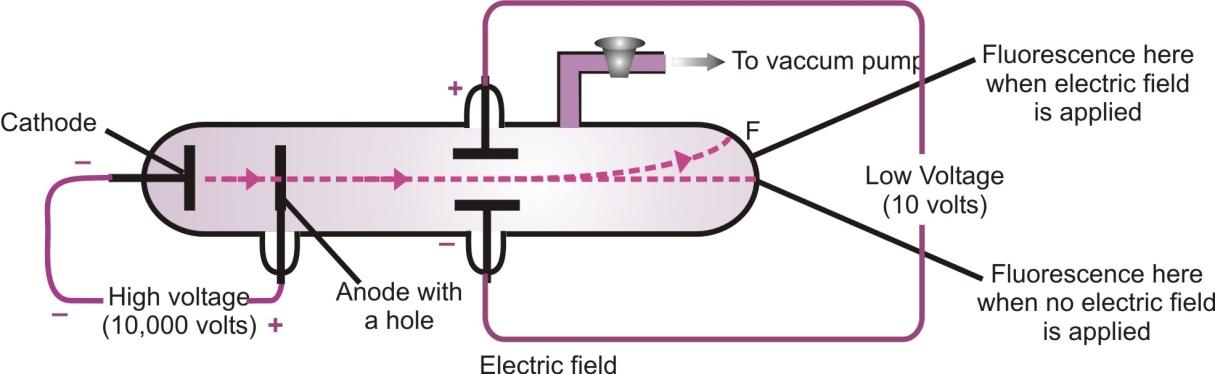
Deflection of cathode rays in electric field
We know that a positively charged body would attract only a negatively charged body, therefore the particles of cathode rays carry negative charge.
Cathode rays also get deflected when these are subjected to a strong magnetic field.
- Cathode rays heat the object only which they fall. The cathode ray particles possess kinetic energy. When these particles strike an object, a part of the kinetic energy is transferred to the object. The causes a rise in the temperature of the object.
- Cathode rays cause green fluorescence on glass surface, i.e., the glass surface only which the cathode rays strike show a colored shine.
- Cathode rays can penetrate through thin metallic sheets.
- Cathode rays ionize the gases through which they travel.
- Cathode rays when fall only certain metals such as copper, but rays produced. The X-rays are not deflected by electrical or magnetic fields. X-rays pass through opaque materials such as black paper, but stopped by solid objects such as bones.
- Cathode rays travel with speed nearly equal to that of light.
Example: What is the use of Thomson Cathode Ray experiment?
Solution: By Cathode ray experiment Thomson come up with the initial idea for the structure of the atom, postulating that It consisted of these negatively charged particles. Swimming in a sea of positive charge. Rutherford then developed this idea further.
Determination of charge and mass of electrons:
Further experiments were carried out to determine the exact charge and mass of electrons.
(i) Charge to mass ratio of electron: J.J. Thomson used different discharge tubes fitted with electrodes of different metals. He studied the extent of deflection of cathode rays under influence of electric fields and magnetic fields of different strengths. He placed different gases in the tube. He found that every time the ratio of charge to mass of the electron was the same. This is usually represented by ‘e/m’, where ‘e’ represents the charge on the electron and m represents the mass of the electrons. The value was found to be:
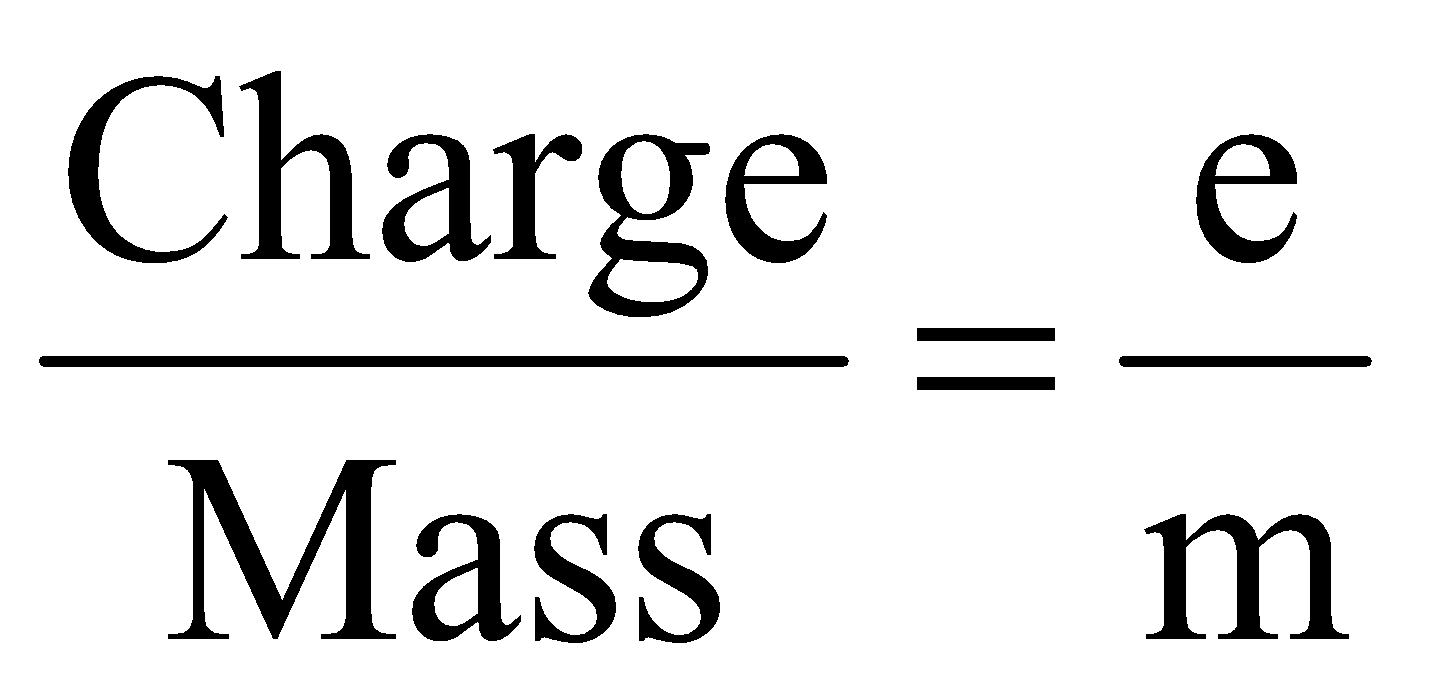 = 1.76 × 108 C/kg (Coulombs/kg)
= 1.76 × 108 C/kg (Coulombs/kg)
Charge on the electron: Charge on the electron was found by R.A. Milliken. He devised a method known as oil drop experiment to determine the charge on the electrons. He found that the charge on the electron was equal to 1.60 × 10–19 C (1 unit).
This is the smallest quantity of charge that could be measured. Hence, it is also called “one unit charge”.
By using the value of e/m and e, the mass of an electron can also be calculated.
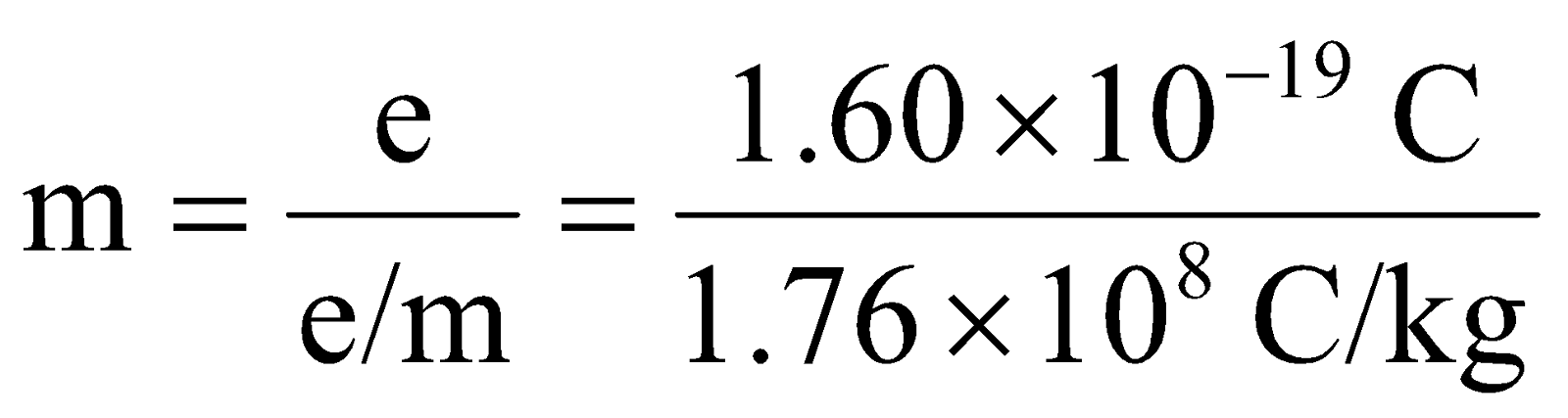
= 9.1 × 10–31 kg
Thus, charge on electron is –1 unit and mass is negligible.

Electrons are constituent of all atoms
Our studies in the discharge tube experiment conducted by J.J. Thomson have shown that we may take electrodes of any material and we may take any gas inside the discharge tube at low pressure. The cathode ray particles have the same e/m ratio as well as the charge (e) i.e. they carry the same charge and mass. This shows that electrons are constituents of all atoms.
DISCOVERY OF PROTON:
The existence of positively charged particles in an atom was shown by Goldstein. Electric discharge experiment carried out in the modified cathode ray tube led to the discovery of particles carrying charge. He took a discharge tube with a perforated cathode and a gas at low pressure was taken inside the discharge tube.
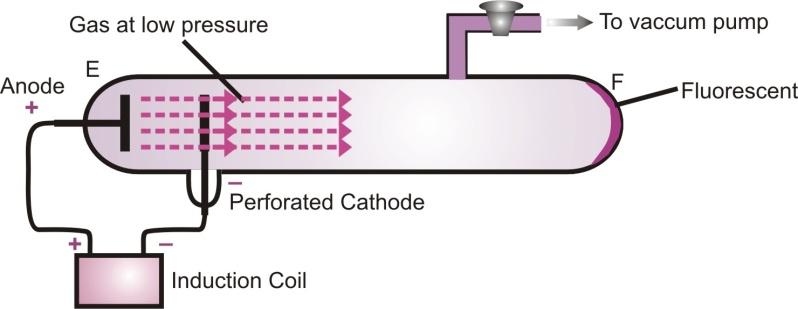
Production of anode rays or canal rays
On applying high voltage between the anode and the cathode, it is observed that like cathode rays produce fluorescence on the glass wall of the tube at E, fluorescence is also observed on the glass wall of the tube F. This shows that some rays are also coming from the anode which passed through the holes in the cathode and strike, the wall of the tube F. These rays are called anode rays, as they are coming from the side of anode. They are also known as canal rays. Their deflection in an electric field indicates that they carry positive charge.
Properties of anode rays / canal rays:
The characteristic properties of the positively charged rays or anode rays or canal rays are listed below:
- They travel in straight lines: If an object is placed in their path, a shadow is produced at the back. This shows that canal rays travel in a straight line.
- They are made up of material particles: Like cathode rays they also rotate the paddle wheel placed in their path. This shows that they are made up of material particles.
- They carry positive charge: In the electric field, these rays are deflected towards the negative plate. It shows that these rays carry positive charge.
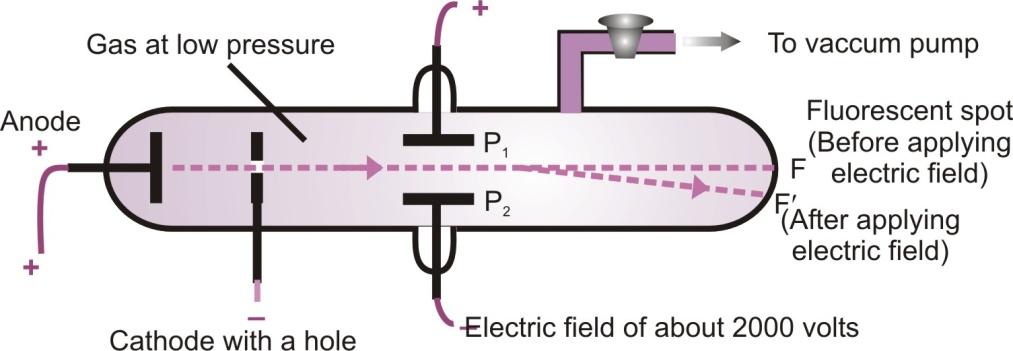
Deflection of anode rays in an electric field
- Determination of charge / mass ratio of the positively charged particles present in anode rays: Unlike cathode rays, the ratio of e/m is found to be different for different gases or we can say that e/m is not constant but depends upon the nature of the gas taken in the discharge tube.
- The value of charge on the particles constituting the anode rays is also dependent on the nature of the gas taken inside the discharge tube.
- Mass of the particle constituting the anode rays is also found to be different for the different gases taken in the discharge tube.
Determination of charge and mass of protons:
The charge and mass of protons are also determined experimentally like that of electrons.
The charge on these particles is found to be same as that on the electrons, i.e.
e = +1.60 × 10–19 C
The ratio of charge / mass (i.e. e/m) = 9.58 × 108 C/kg
The mass of proton  = 1.67 × 10–27 kg
= 1.67 × 10–27 kg
This mass is nearly the same as that of hydrogen atom. When hydrogen gas is used in the discharge tube, the particles constituting the anode rays are called protons. Hence, a proton can be defined as follows:
“That sub-atomic or fundamental particle carrying one unit positive charge and has mass nearly equal to that of hydrogen atom”
As charge on proton is +1 and mass = 1u, hence, it may be represented by the symbol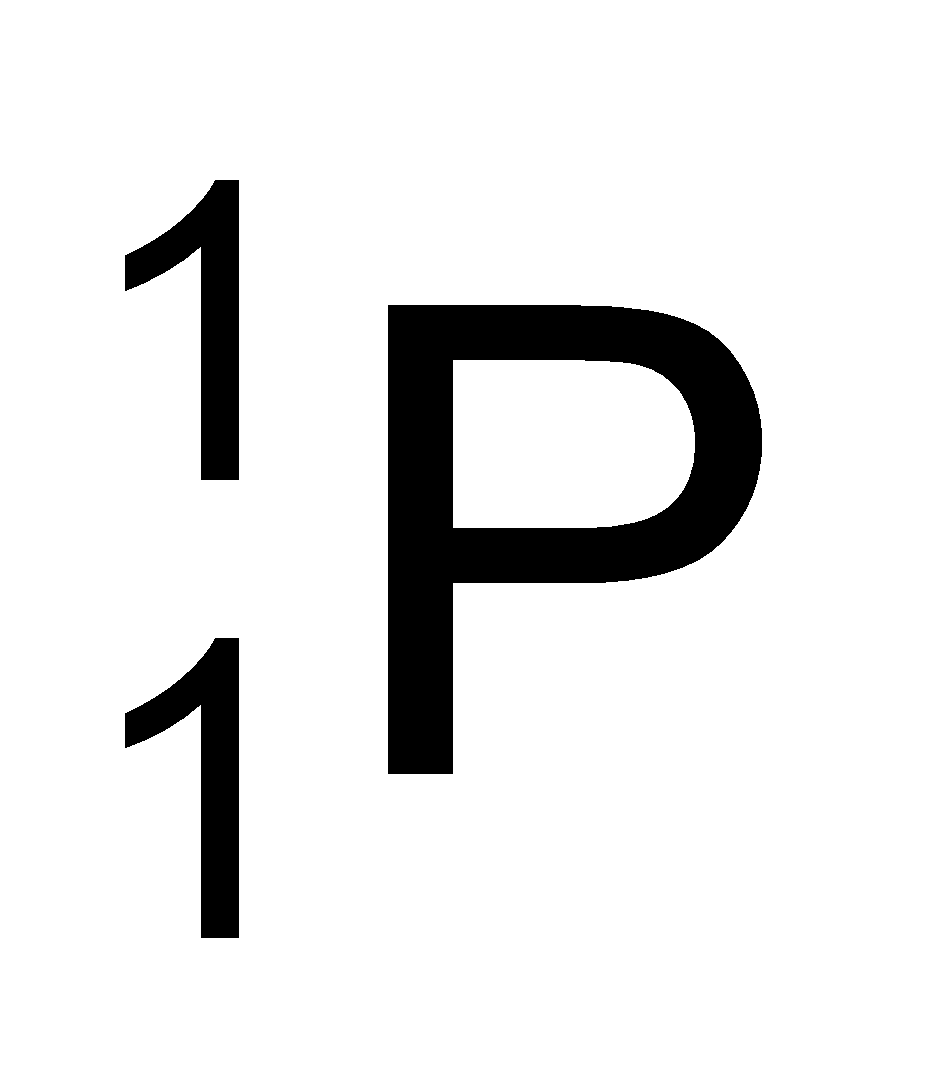 .
.
Protons are constituents of all atoms
If any other gas (other than hydrogen) is taken in the discharge tube, it is observed that the mass of positively charged particle is nearly a whole number multiple of the mass of proton. Hence, it can be concluded that protons are the fundamental particles present in all atoms.
DISCOVERY OF NEUTRON:
In 1920, Ernest Rutherford conceived the possible existence of the neutron. In particular, Rutherford considered that the disparity found between the atomic number of an atom and its atomic mass could be explained by the existence of a neutrally charged particle within the atomic nucleus. He considered the neutron to be a neutral double consisting of an electron orbiting a proton.
In 1930 Viktor Ambartsumian and Dmitri Ivanenko in USSR found that, contrary to the prevailing opinion of the time, the nucleus cannot consist of protons and electrons. They proved that some neutral particles must be present besides the protons.
In 1931, Walther Bothe and Herbert Becker in Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris. They showed that if this unknown radiation fell on paraffin, or any other hydrogen-containing compound, it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis.
In 1932, James Chadwick performed a series of experiments at the University of Cambridge, showing that the gamma ray hypothesis was untenable. He suggested that the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion. These uncharged particles were called neutrons, apparently from the Latin root for neutral and the Greek ending -on (by imitation of electron and proton).
Comparison of the characteristics of electron, proton and neutron.
|
Particle |
Charge on the particle |
Mass of the particle |
Symbol |
Location in the atom |
|
1. Electron |
− 1 unit (−1.602 × 10−19 C) |
9.11 × 10−31 kg
|
|
Outside the nucleus (Extra nuclear part) |
|
2. Proton |
+ 1 unit (+1.602 × 10−19 C) |
1.673 × 10−27 kg
|
|
In the nucleus |
|
3. Neutron |
No charge |
1.675 × 10−27 kg
|
|
In the nucleus |


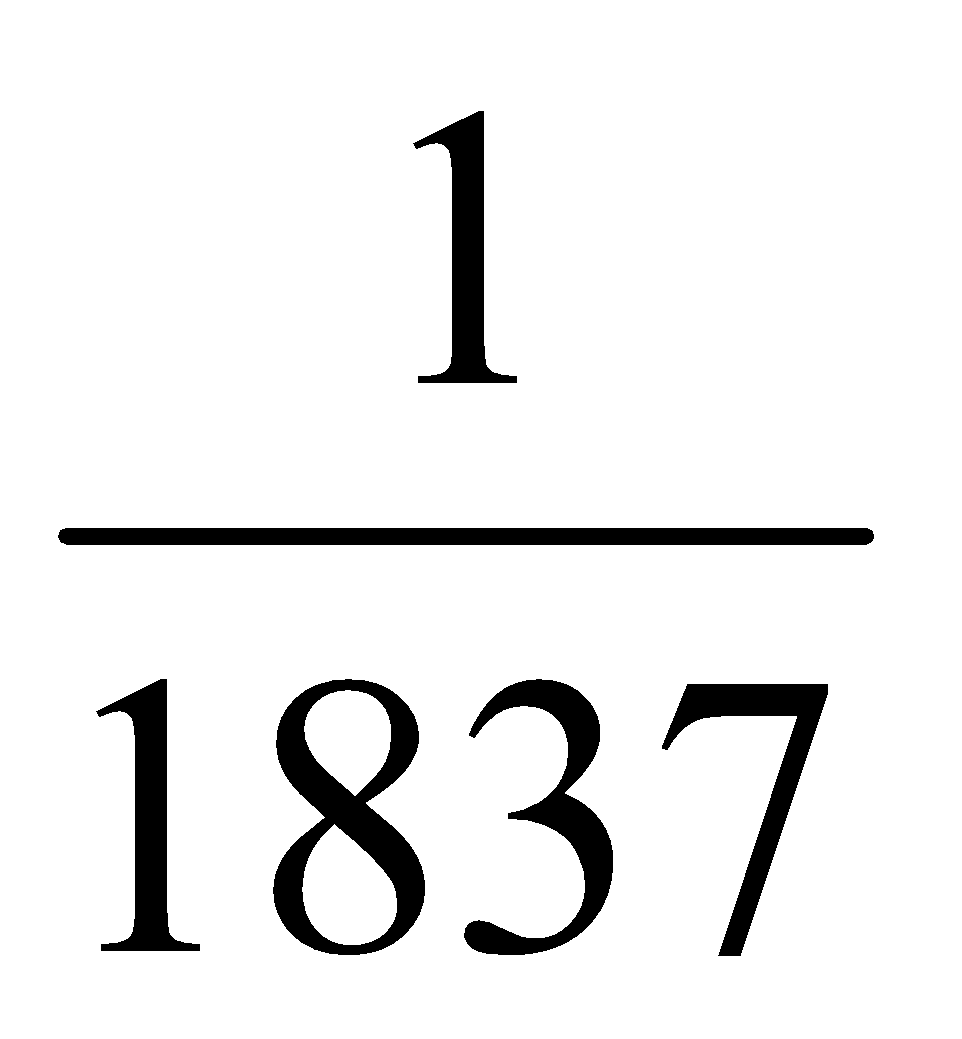
.jpg)
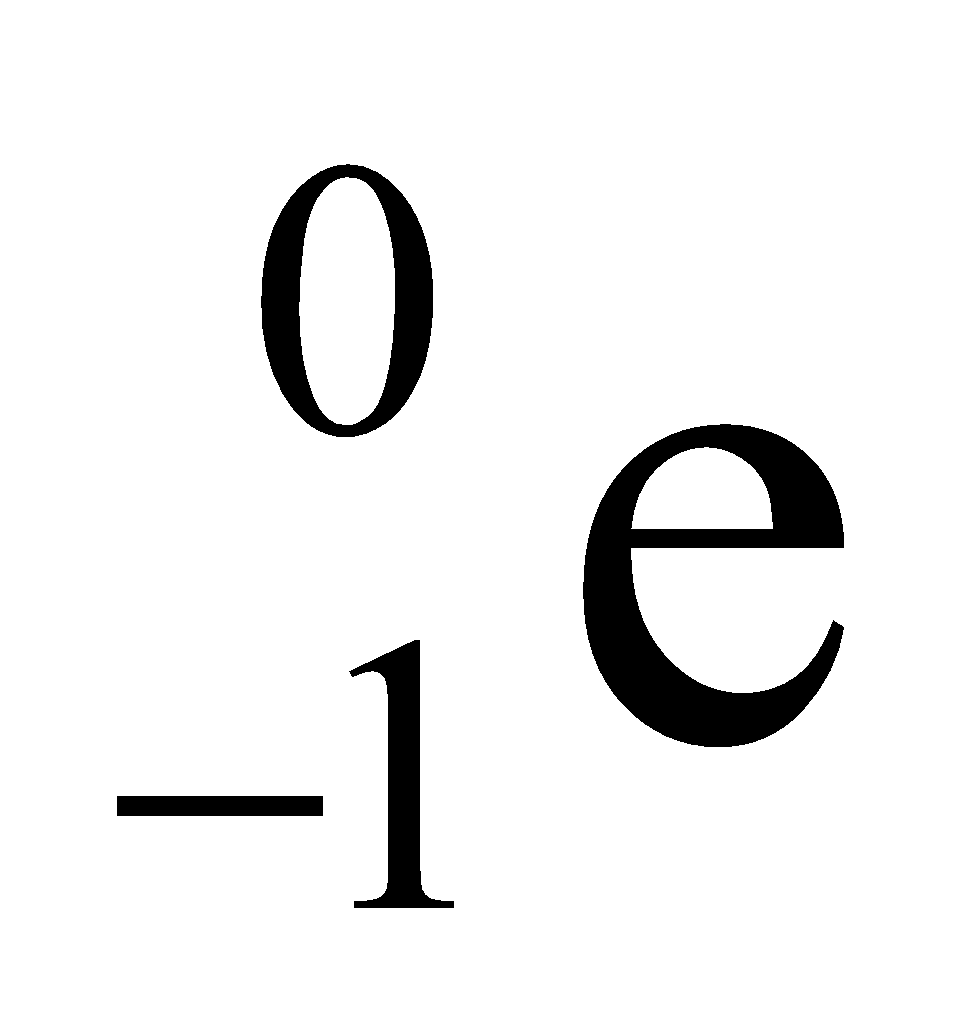
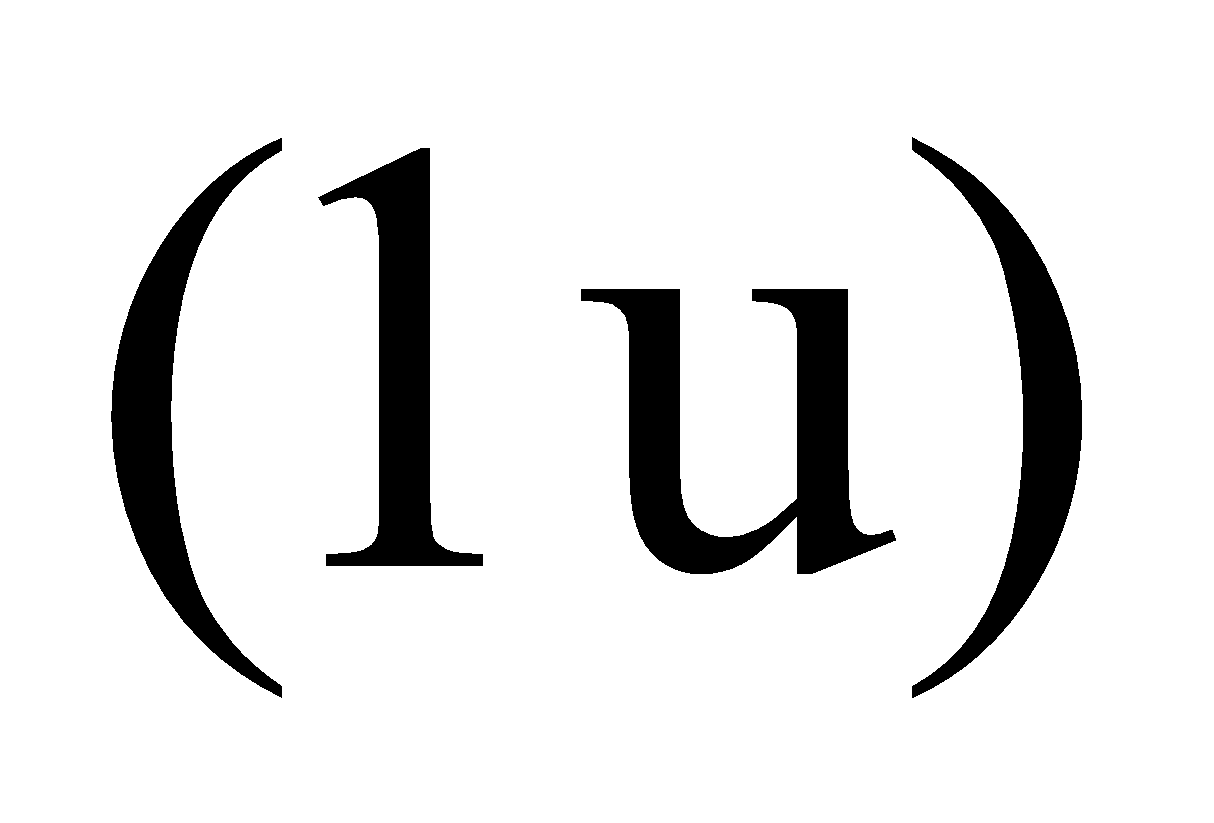
.jpg)
.jpg)