THE STRUCTURE OF AN ATOM (ATOMIC MODELS)
Structure of atom of Class 9
THE STRUCTURE OF AN ATOM (ATOMIC MODELS)
According to Dalton’s atomic theory, an atom is indivisible and indestructible. But after the discovery of subatomic particles (electrons, protons and neutrons), various atomic models were proposed by many scientists to explain their arrangement in the atom.
THOMSONS MODEL OF AN ATOM
After the discovery of electrons and protons J.J. Thomson (1898) tried to explain the arrangement of electrons and protons within the atom. He proposed that an atom consists of a sphere of positive electricity in which electrons are embedded like plum in pudding or seeds evenly distributed in red spongy mass in watermelon. The radius of the sphere is of the other 10-8 cm which is equal to the size of the atom. Although Thmoson’s model could explain the electrical neutrality of an atom but this model could not satisfy experimental facts proposed by Rutherford and hence was discarded.
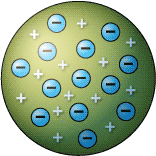
Plum pudding model
|
|
“Proton is a sub - atomic particle having a unit positive charge (+1.602 × 10-19 kg) & mass (1.6725 × 10-27 kg) which is about 1837 times greater than the mass of an electron.” |
RUTHERFORD’S MODEL OF AN ATOM:
(a) Rutherford’s Alpha Scattering Experiment :
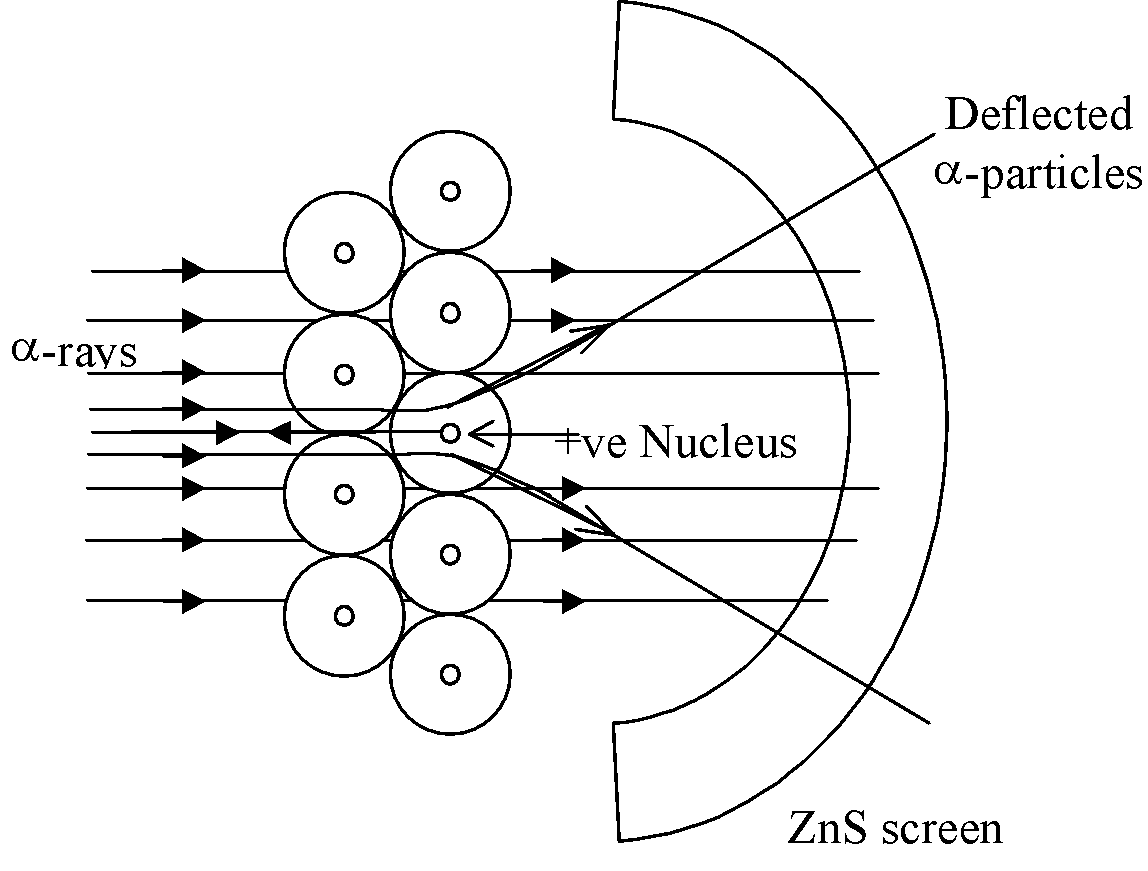
Ernest Rutherford and his co-workers performed numerous experiments in which 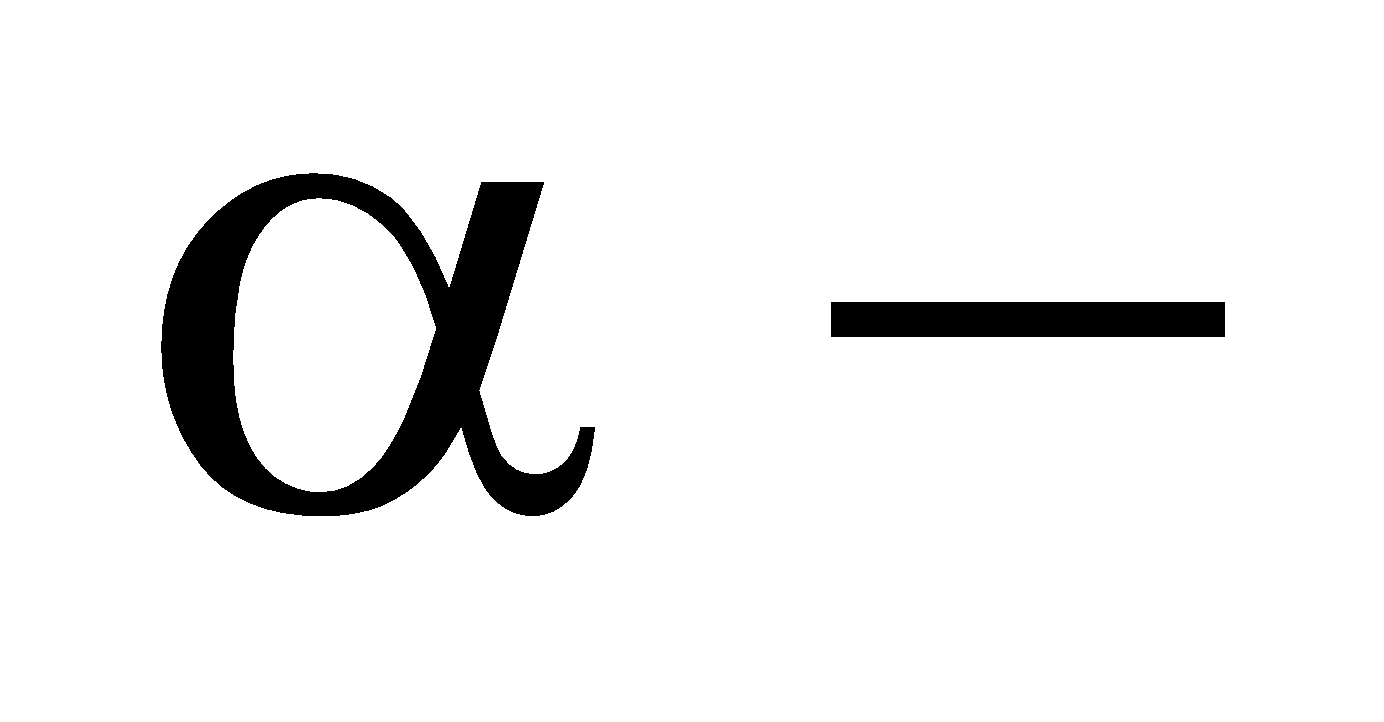 particles emitted from a radioactive element such as polonium were allowed to strike thin sheets of metals such as gold and platinum.
particles emitted from a radioactive element such as polonium were allowed to strike thin sheets of metals such as gold and platinum.
-
A beam of
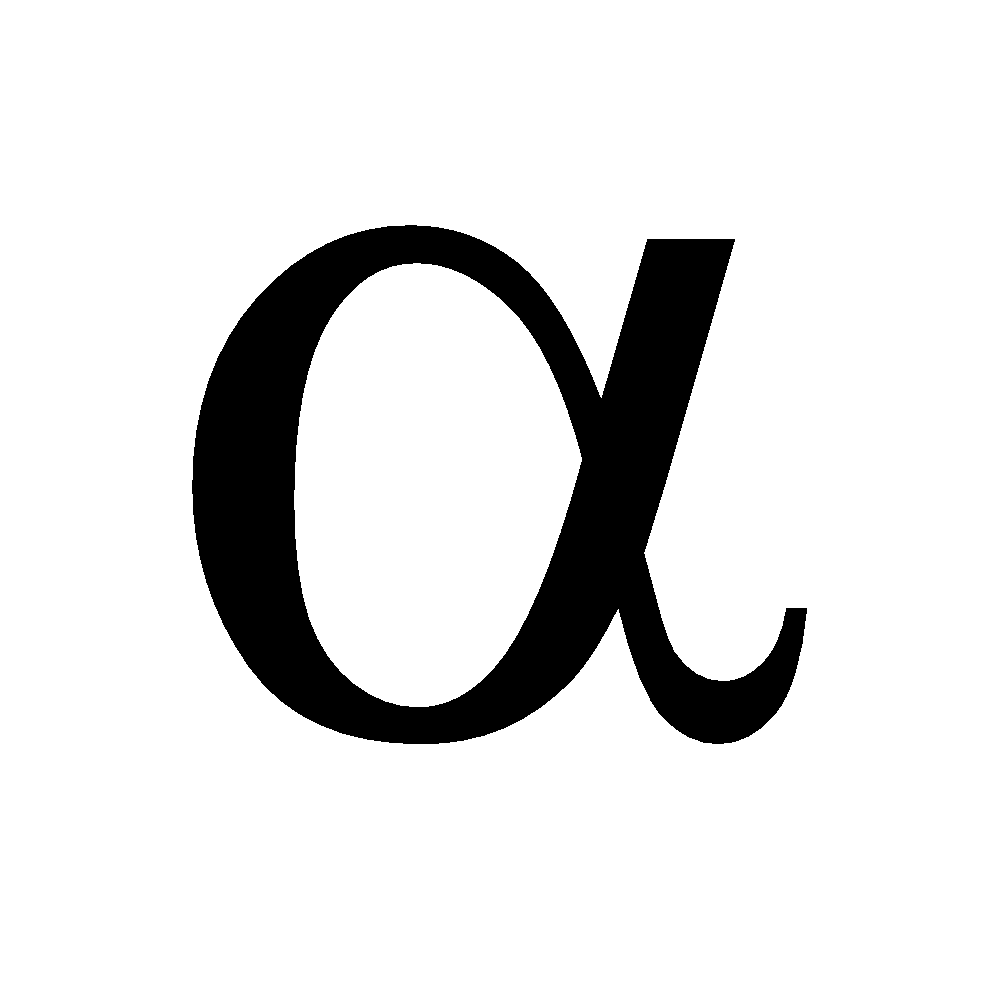 -particles (He2+) we obtained by placing polonium in a lead box and letting the alpha particles come out of a pinhole in the lead box. This beam of
-particles (He2+) we obtained by placing polonium in a lead box and letting the alpha particles come out of a pinhole in the lead box. This beam of  -rays was directed against a thin gold foil (0.00004 cm). A circular screen coated with zinc sulphide was placed on the other side of the foil.
-rays was directed against a thin gold foil (0.00004 cm). A circular screen coated with zinc sulphide was placed on the other side of the foil. - About 99% of the α– particles passed undeflected through the gold foil and caused illumination of zinc sulphide screen.
-
Very few
 -particles underwent small and large deflections after passing through the gold foil.
-particles underwent small and large deflections after passing through the gold foil. - A very few (about 1 in 20,000) were deflected backward on their path at an angle of 180o.
Observations: After the bombardment of α-particles on the thick gold foil, Rutherford observed that:
- Most of the fast moving α-particles passed through the gold foil undeflected.
- Some of the α-particles were deflected by small angles and some were deflected through large angles.
- A very few particles (1 in 20,000) bounded back i.e. were deflected by nearly 180°.
Conclusion: On the basis of these observations, Rutherford drew the following conclusions regarding the structure of atom :
- Most of the space in an atom is empty as most of the α-particles passed through the foil undeflected.
- A few α-particles were deflected from their path. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom, as Thomson had thought. According to Rutherford, the positive charge of the atom occupies very little space. This very small portion of the atom was called nucleus.
- A very small fraction of the α-particles were deflected by 180°, showing that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom. (Radius of the atom is about 10–10 m while that of nucleus is 10–15 m).
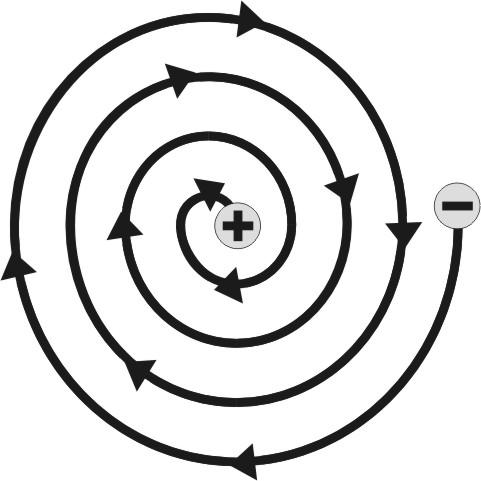
Rutherford’s Nuclear Model of Atom:
Rutherford proposed a new picture of the structure of the atom. Main feature of this model are as follows -
- The atom of an element consists of a small positively charged “Nucleus” which is situated at the centre of the atom and which carries almost the entire mss of the atom.
- The electrons are distributed in the empty space of the atom around the nucleus in different concentric circular paths (orbits).
- The number of electrons in the orbits is equal to the total number of protons in the nucleus.
- Volume of nucleus is very small as compared to the volume of atom.
- Most of the space in the atom is empty.
The stability of such a system in which negatively charged electrons surround a positively charged nucleus was explained by proposing that the electrons revolve around the nucleus at very high speed in circular orbits. This arrangement is just like our solar system. The high sped of the moving electrons given them a centripetal force acting away from the nucleus. The centripetal force balances the electrostatic force of attraction acting between the nucleus and the electrons.

Drawbacks of Rutherford’s Model of an atom:
|
Rutherford’s model could not explain the stability of an atom. This is because when a particle is moving in a circular orbit, it undergoes acceleration. During acceleration charged particles would radiate energy. Thus, the orbit of the revolving electrons will keep on shrinking or becoming smaller and smaller, following a spiral path and will ultimately fall into the nucleus. However, this actually does not happen and we know that atoms are quite stable. |
|
BOHR’s MODEL OF AN ATOM:
Rutherford’s model of the atom was unable to explain certain observations with regard to the atom i.e. stability of the atom and the occurrence of the atomic spectra. Neils Bohr accepted Rutherford’s idea that the positive charge and most of the mass of the atom is concentrated in its nucleus with the electrons present at some distance away.
According to Bohr’s theory -
- Electrons revolve around the nucleus in well defined orbits or shells, each shell having a definite amount of energy associated with the electrons in it. Therefore, these shells are also called energy levels.
- The energy associated with the electrons in an orbit increases as the radius of the orbit increases. These shells are known as K, L, M, N etc. starting from the one closest to the nucleus.
- An electron in a shell can more to a higher or lower energy shell by absorbing or releasing a fixed amount of energy.
- The amount of energy absorbed or emitted is given by the difference of energies associated with the two energy levels.
Energy absorbed, ΔE = E2 – E1 = 
Energy emitted, ΔE = E2 – E1 = 
Where h is Plank’s constant (h = 6.62 × 10-34 Js) and  is the frequency of the radiation.
is the frequency of the radiation.