MASS NUMBER (A)
Structure of atom of Class 9
MASS NUMBER (A)
Mass number is the number of protons and neutrons present in the atom of an element. It is denoted by “A”. The mass number is represented either on the left hand side (LHS) or on the right hand side (RHS) of the symbol of the element as superscript.
Mass number = Number of protons + Number of neutrons.
e.g. 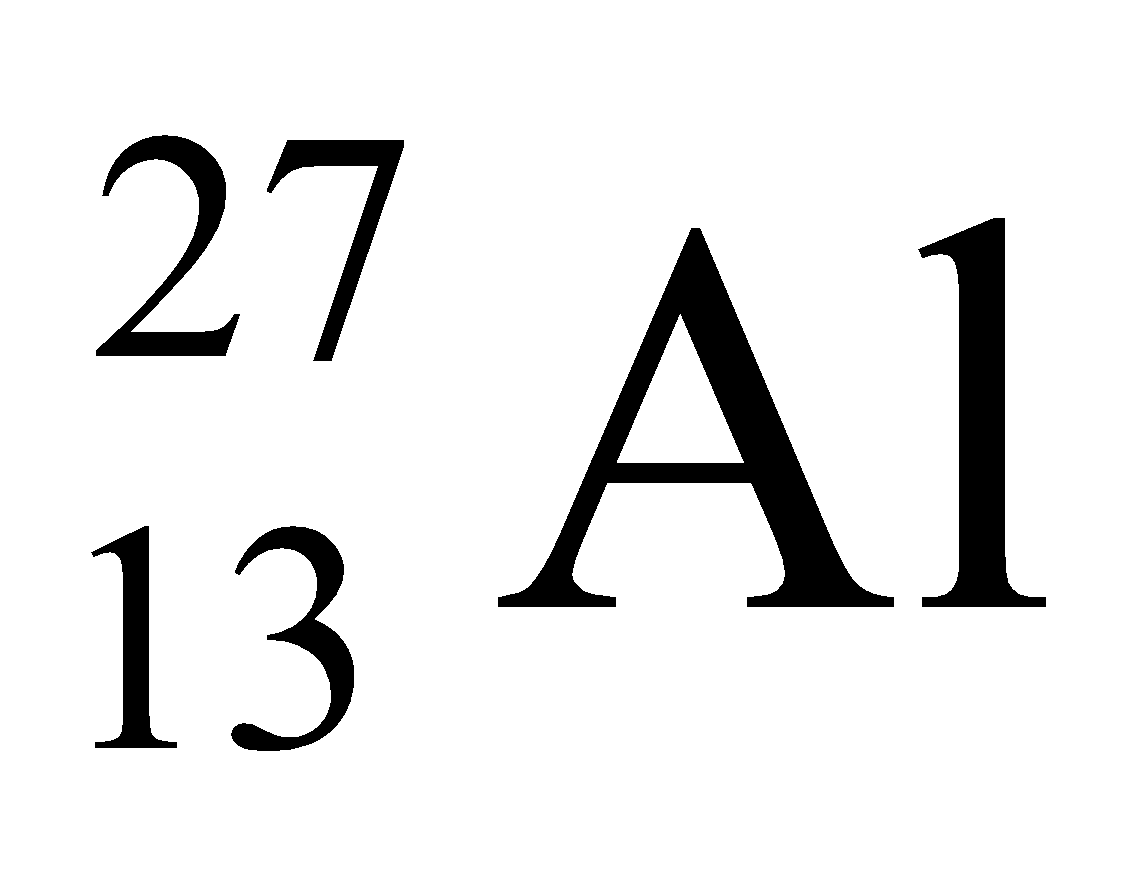
- Mass number of aluminum is 27.
- The total number of protons and neutrons is the nucleus of aluminum is 27.
- Number of protons is 13.
- Number of neutrons is = 27 - 13 = 14.
(a) Relation between Z, A and N
A = Z + N
Z = Number of Protons
N = Number of neutrons
A = Mass number
 N = A - Z
N = A - Z
Ex. 1 If number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom?
(ii) What is the charge on the atom?
Solution: (i) Atomic number (Z) = No. of protons = No. of electrons = 8
(ii) As number of protons and electrons are equal hence the atom will be neutral i.e. there will be no charge on the atom.
Ex. 2 Find out the mass number (A) of oxygen and sulphur.
[Given: Number of protons = electrons = neutrons = 8 for oxygen atom and number of protons = neutrons = 16 for sulphur.]
Solution: (i) Mass number of Sulphur = n + p = 16 + 16 = 32
(ii) Mass number of oxygen = n + p = 8 + 8 = 16
Ex. 3 An atom consists of 40 protons, 45 neutrons and having +2 charge, then find atomic mass, atomic number and number of electrons.
Solution: P + N = Atomic mass
So, Atomic mass = 85
As it consists of +2 charge that means total number of electrons are 38 electrons
Atomic number = number of protons = 40
- Introduction
- FUNDAMENTAL PARTICLES OF AN ATOM
- VALENCY AND VALANCE ELECTRONS
- ISOTOPES
- ISOELECTRIC
- DISCOVERY OF ATOM
- SUB-ATOMIC PARTICLES OF ATOM
- THE STRUCTURE OF AN ATOM (ATOMIC MODELS)
- ATOMIC STRUCTURE
- ORBITALS
- ATOMIC NUMBER (Z)
- MASS NUMBER (A)
- what is isobar
- what is isotones
- Solved questions
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5
- Exercise 6 (True and False)









