what is isobar
Structure of atom of Class 9
ISOBARS
“Atoms of different elements which have different atomic number but same mass number are called isobars. They have different number of protons, electrons and neutron but the mass number, i.e. the sum of protons and neutrons in the nucleus is same”
Example:  ,
, 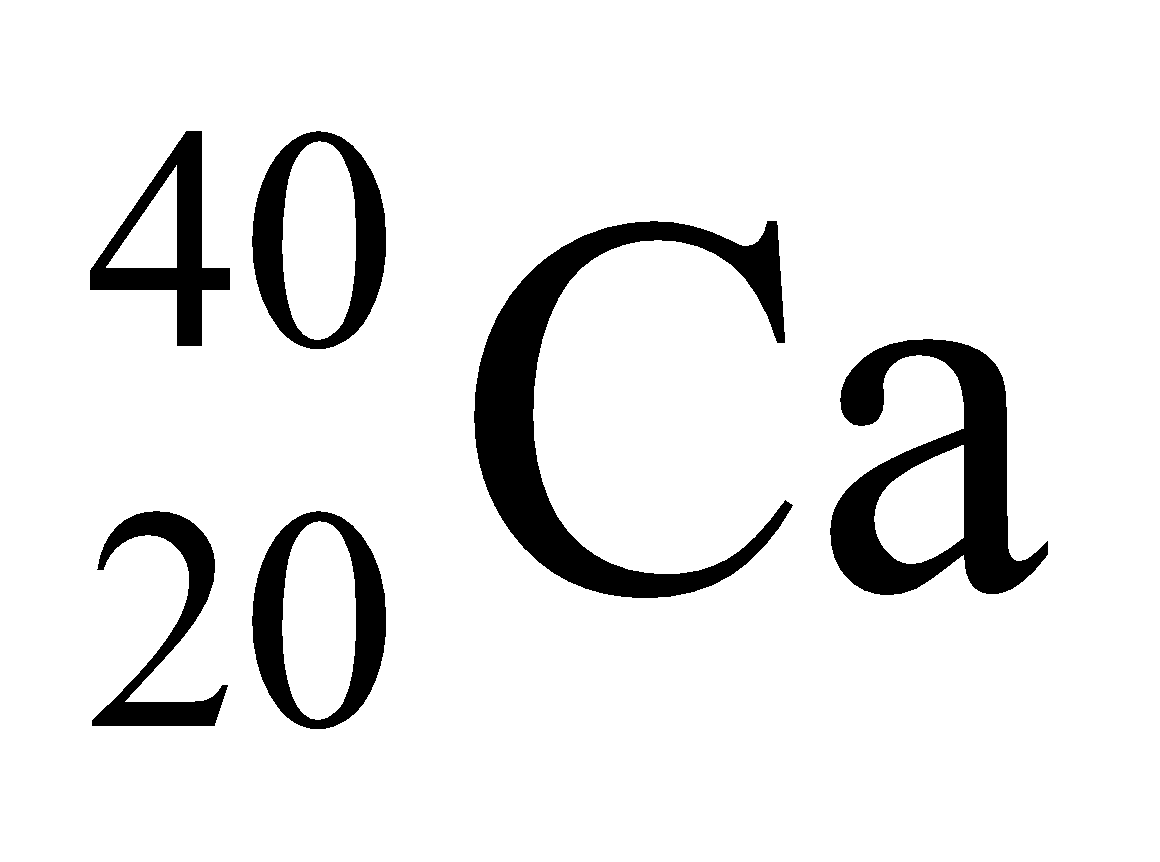
|
Isobars |
Protons |
No. of electrons |
No. of neutrons |
|
|
18 |
18 |
40–18 = 22 |
|
|
20 |
20 |
40–20 = 20 |
General Characteristics of Isobars:
- They are atoms of different elements.
- They have different atomic number.
- They possess different physical as well as chemical properties.
- Introduction
- FUNDAMENTAL PARTICLES OF AN ATOM
- VALENCY AND VALANCE ELECTRONS
- ISOTOPES
- ISOELECTRIC
- DISCOVERY OF ATOM
- SUB-ATOMIC PARTICLES OF ATOM
- THE STRUCTURE OF AN ATOM (ATOMIC MODELS)
- ATOMIC STRUCTURE
- ORBITALS
- ATOMIC NUMBER (Z)
- MASS NUMBER (A)
- what is isobar
- what is isotones
- Solved questions
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5
- Exercise 6 (True and False)

.jpg)









