
ORBITALS
Structure of atom of Class 9
ORBITALS
Like shells are divided into subshells, subshells further contain orbitals.
An orbital may be defined as a
“Region in the three - dimensional space around the nucleus where the probability of finding an electron is maximum. The maximum capacity of each orbital is that of two electrons.”
|
Sub shell |
Orbital (s) |
Max. no. of electrons |
|
s |
1 |
2 |
|
p |
3 |
6 |
|
d |
5 |
10 |
|
f |
7 |
14 |
The total number of nucleons is equal to the mass number (A) of the atom.
BOHR-BURY SCHEME OF DISTRIBUTION OF ELECTRONS:
The following rules are given by Bohr and Bury for writing the number of electrons in different energy levels or shells:
(i) The maximum number of electrons that can be present in a given shell is equal to 2n2, where n = number of shell.
Hence, the maximum number of electrons in different shells can be given as follows:
|
Shell |
Maximum No. of electrons present |
|
(a) 1st shell or K-shell (n = 1) |
2 × (1)2 = 2 |
|
(b) 2nd shell or L-shell (n = 2) |
2 × (2)2 = 8 |
|
(c) 3rd shell or M-shell (n = 3) |
2 × (3)2 = 18 |
|
(d) 4th shell or N-shell (n = 4) |
2 × (4)2 = 32 |
- The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons do not enter into a new shell until and unless the inner shells are completely filled or we can say that shells are filled in a step-wise manner.
|
Element |
Symbol |
Atomic No. (No. of electrons) |
Distribution of electrons in different shells |
Short representation of electronic configuration |
|||
|
K |
L |
M |
N |
||||
|
Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulphur Chlorine Argon Potassium Calcium |
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca |
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 |
1 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 |
1 2 3 4 5 6 7 8 8 8 8 8 8 8 8 8 8 8 |
1 2 3 4 5 6 7 8 8 8 |
1 2 |
1 2 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8 2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2, 8, 5 2, 8, 6 2, 8, 7 2, 8, 8 2, 8, 8, 1 2, 8, 8, 2 |
Diagrammatically, the nuclear structure and the distribution of electrons can be represented as below:
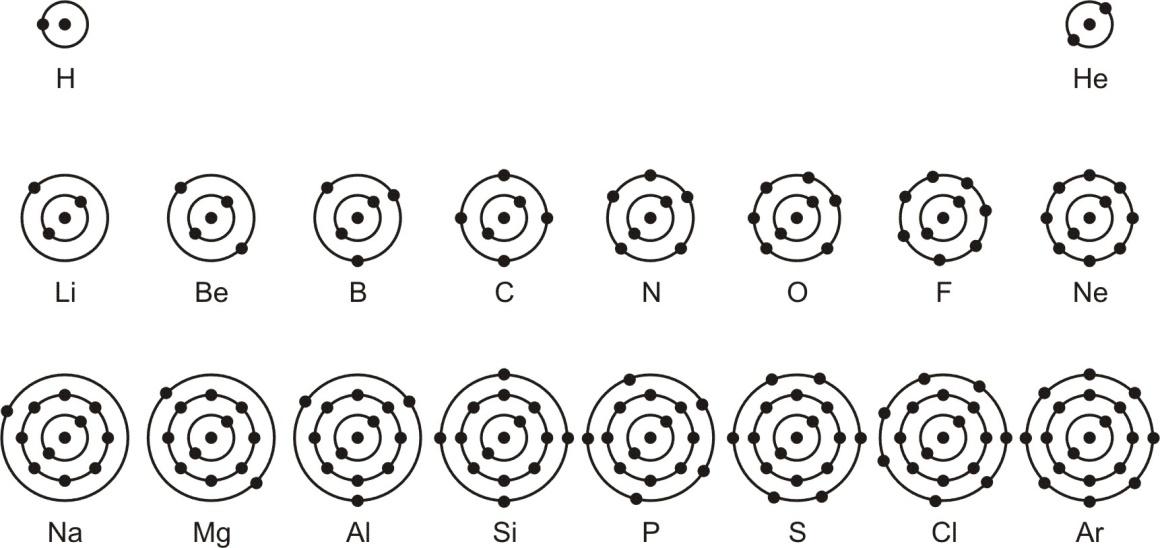
Schematic atomic structure of the first eighteen elements
