
Dalton's Atomic Theory
Atomic Structure of Class 10
DALTON’S ATOMIC THEORY
On the basis of large number of experiments performed, John Dalton proposed that matter was made up of extremely small particles called atoms.
The main postulates of Dalton’s atomic theory are:
- Matter is made up of small indivisible particles called atom.
- Atom is the smallest particle of an element, which takes part in a chemical reaction.
- Atoms of the same element are identical in all respects especially in size, shape and mass.
- Atoms of different elements have different mass, shape and size.
- Atoms can neither be created nor be destroyed. This means that a chemical reaction is just a simple rearrangement of atoms and the same number of atoms must be present before and after the reaction.
- Atoms of different elements combine in a fixed ratio of small whole numbers to form compound atoms called molecules.
However, the researches done by various eminent scientists and the discovery of radioactivity have established beyond doubt that atom was not the smallest indivisible particle but has a complex structure of its own and was made up of still smaller particles like electrons, protons, neutrons etc. At present about 35 different subatomic particles are known but the three particles namely electron, proton and neutron are regarded as the fundamental particles.
Demerits of Dalton’s Atomic theory:-
Dalton’s atomic theory has been contradicted with the advancement of science and modified on the basis of further research and discoveries as follows:
- With the discovery of subatomic particles, i.e. the electron, proton, and neutron, it was concluded that atom can be further divided.
- Discovery of isotopes proved that atoms of the same element may possess different atomic weights. i.e. atoms of same elements may not be identical in all respect.
Though Dalton’s atomic theory could not give a convincing explanations to any of the above facts, it laid the foundation for the development of Modern atomic theory.The basic postulate of Dalton’s atomic theory which says that “Atom’s are the tiniest particles of matter which take part in Chemical reaction” is however accepted in modern atomic theory with experimental evidence.
DISCOVERY OF ELECTRON: CATHODE RAYS
During the latter half of the nineteenth century, it was found that while normally dry gases do not conduct an electric current, they do so under very low pressure and then patches of light are seen. The passage of electricity through gases as studied by a number of physicists, particularly by Faraday, Davy, Crookes and J.J. Thomson.
When a current of high voltage (10,000 volts) is passed through a gas of air kept at a very low pressure (0.01 – 0.03 mm) blue rays are seen emerging from the case. These rays are called “Cathode Rays”.
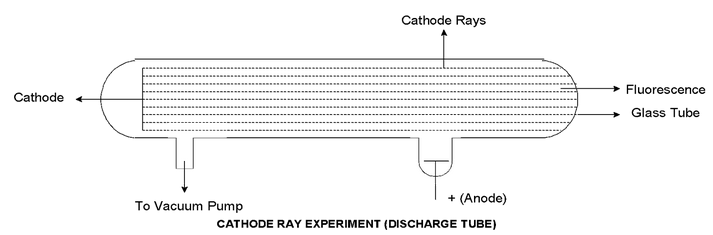
Some of the important properties of the cathode rays studied by Sir J.J. Thomson and others are given below:
- Cathode rays come out at right angles to the surface of the cathode and move in straight lines.
- Their path is independent on the position of the anode.
- They produce phosphorescence on certain salts like ZnS and fluorescence on glass.
- They blacken photographic plates.
- The rays pass through thin sheet of metals. If the metal sheet is too thick to be penetrated the rays cast a shadow.
- They produce X−ray when they strike a metal.
- The rays ionize a gas through which they pass.
- They heat a substance on which they fall.
- They rotate a light wheel placed in their paths. This shows that cathode rays contain material particles having both mass and velocity.
- The mass of a particle present in cathode rays is found to be 1/1837 of H−atom. This shows that the particle is of sub−atomic nature.
- Cathode rays are deflected by a magnetic or an electric field showing the particle to be electrically charged, the direction of deflection shows that they are negatively charged.
- Cathode rays contain the smallest unit of negative charge.
- No cathode ray was produced when the tube was completely evacuated.
- Different gases produce same cathode rays as they have the same e/m (charge/mass) ratio. This indicates that the particles present in cathode rays are fundamental constituent of all matter.
Sir J.J. Thomson named these negatively charged sub−atomic particles as electron. “A sub−atomic particle which is a fundamental constituent of all matter having a mass 1/1837th of a H−atom and which carries the smallest unit of negative charge is called an electron”.
DISCOVERY OF PROTON: POSITIVE RAYS OR CANAL RAYS
Atoms are electrically neutral. Hence after the discovery of the negatively charged constituent (electron) of an atom, attempts were made to discover the positively charged counterpart of electrons. By using a discharge tube containing a perforated cathode. Goldstein (1886) found that some rays passed through these holes in a direction opposite to that of the cathode rays.
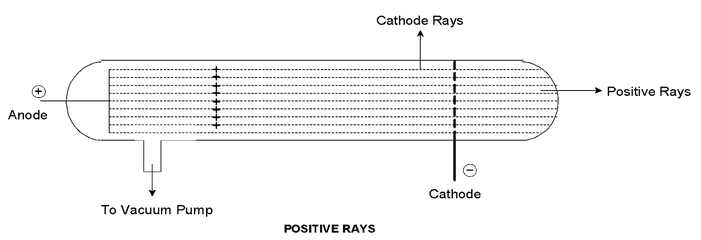
These are called the positive rays or canal rays. J.J. Thomson (1910) measured their charge by mass ratio from which he was able to deduce that these contain positive ions. Their properties are:
- They are positively charged.
- The positive charge is either equal to or whole number multiple of the charge on an electron.
- When hydrogen gas was filled in the discharge tube the positive charge on the positive rays was equal to the negative charge on an electron, and the mass was less than the hydrogen atom.
- Unlike cathode rays the properties of positive rays are characteristics of the gas in the tube.
- The deflection of positive rays under the influence of an electric or magnetic field is smaller than that of the cathode rays for the same strength of field. This shows that the positive rays have a greater mass than that of electrons.
- The mass of the positive rays depends on the atomic weights or molecular weights of the gases in the discharge tube. The charge/mass ratio also varies because the change in positive charge on the rays. It may be either equal to or integral multiple of the charge on an electron.
- The lightest of all particles identified in positive rays from different elements was one with a mass very slightly less than that of hydrogen atom (or nearly equal to H−atom). The lightest positively charged particle is called a proton (P or P+). Positive rays are atomic or molecular resides from which some electrons have been removed. The removed electrons constitute the cathode rays and the positive residues form the positive or canal rays.
|
Positive Rays |
Cathode Rays |
|
|
H |
H+ |
e– |
|
O → |
O+ |
e– |
|
O2→ |
O2+ |
e– |
|
O2 → |
O22+ |
2e– |
The mass of a proton is very slightly less than that of a H−atom. This shows that protons are sub−atomic particles. Protons are fundamental constituent of matter because positive rays are produced by all substances.
“A sub−atomic particle, which is a fundamental constituent of all matter having a mass slightly less than that of H−atom and which carries a positive charge equal in magnitude to the charge on an electron, is called a proton”. A proton is denoted by p or p+ of +1p.
Comparison of Positive (Canal) Rays and Cathode Rays:
|
Properties |
Cathode Rays |
Canal Rays |
|
Sign of Charge |
Negative |
Positive |
|
Mag. of Charge |
Always –1 |
Mostly +1, but also +2, +3… |
|
Mass |
Definite value |
Variable, depends on ions |
|
e/m |
Definite value |
Variable, depends on ions |
DISCOVERY OF NEUTRON
After the discovery of electrons and protons. Rutherford (1920) had predicted the existence of a neutral fundamental particle. In 1932, Chadwick bombarded the element Beryllium with α−particles and noticed the emission of a radiation having the following characteristics.
- The radiation was highly penetrating.
- The radiation was unaffected by magnetic and electric fields which show that it is electrically neutral.
- It was found to have approximately the same mass as the protons.
The name ‘neutron’ was given to this sub−atomic particle. It is denoted by n or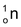 . Bombardment of beryllium by α−particles results in the formation of carbon and neutrons are emitted.
. Bombardment of beryllium by α−particles results in the formation of carbon and neutrons are emitted.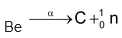
At present there are a number of evidences which confirm that like electron, proton and neutron is also a fundamental constituent of atoms (a single exception is 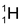 atom which does not contain any neutron)
atom which does not contain any neutron)
Mass of a neutron is 1.008930 amu (1.6753 × 10–24g or 1.6753 × 10–27 kg)
Neutron “A sub−atomic particle, which is a fundamental constituent of matter having mass approximately equal to the hydrogen atom and which is electrically neutral, is called a neutron”.
Characteristic of Fundamental Particles
|
Electron |
Proton |
Neutron |
|
|
Symbol |
e- |
p |
n |
|
Approximate relative mass |
1/1836 |
1 |
1 |
|
Approximate relative charge |
-1 |
+1 |
0 |
|
Mass in kg |
9.109534 × 10-31 |
1.6726485 × 10-27 |
1.6749543 × 10-27 |
|
Mass in amu |
5.4858026 × 10-4 |
1.007276471 |
1.008665012 |
|
Actual charge/C |
1.6021892 × 10-19 |
1.6021892 × 10-19 |
0 |

