Models Of Atom
Atomic Structure of Class 10
MODELS OF ATOMS
As it was experimentally well established that atom consists of different particles, there have been attempts to present different models of atom.
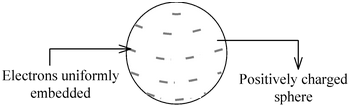
Plum Pudding Model of Thomson
|
After the discovery of electrons and protons, Thomson gave a model called Plum Pudding Model. He suggested that atom is a positively charged sphere having electrons embedded uniformly giving an overall picture of plum pudding. |
|
Rutherford Model
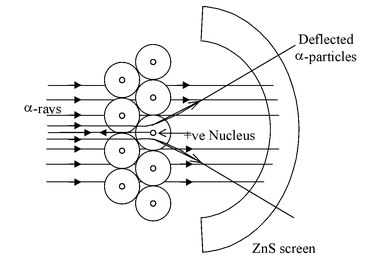
ExperimentRutherford allowed a narrow beam of α-particles to fall on a very thin gold foil of thickness of the order of 0.0004 cm and determined the subsequent path of these particles with the help of a zinc sulphide fluorescent screen. The zinc sulphide screen gives off a visible flash of light when struck by an α particle, as ZnS has the remarkable property of converting kinetic energy of α particle into visible light. |
|
Observations
- Majority of the α-particles pass straight through the gold strips with little or no deflection.
- Some α-particles are deflected from their path and diverge.
- Very few α-particles are deflected backwards through angles greater than 90°.
- Some α-particles were even scattered in the opposite direction at an angle of 180° [Rutherford was very much surprised by it and remarked that “It was as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you”].
Atomic Model Proposed by Rutherford
On the basis of the above observations, Rutherford proposed an atomic model as follows
- Atom consists of two parts: (a) nucleus and (b) extra nuclear part.
- All the protons (+ve charge) and the neutrons (neutral charge) i.e. nearly the total mass of an atom is present in very small region at the centre of the atom. The atom’s central core is called nucleus.
- The size of the nucleus is very small in comparison to the size of the atom. Diameter of the nucleus is about 10-13 cm while the atom has a diameter of the order of 10−8 cm. So, the size of atom is 105 times more than that of nucleus.
- Most of the space outside the nucleus is empty.
- The electrons (equal in number to the net nuclear positive charge) revolve around the nucleus with fast speed in various circular orbits.
- The centrifugal force arising due to the fast speed of an electron balances the coulombic force of attraction of the nucleus and the electron remains stable in its path.
Defects of Rutherford’s Atomic Model
1. Position of electrons: The exact positions of the electrons from the nucleus are not mentioned.
|
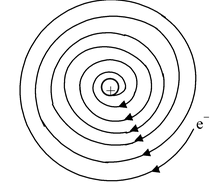
2. Stability of the Atom: Neils Bohr pointed out that Rutherford’s model of atom is defective. According to the law of electro-dynamics, the electron should therefore, continuously emit or use energy for moving and get closer to the nucleus and after passing through a spiral path, ultimately fall into the nucleus. |
|
To solve this problem Neils Bohr proposed an improved form of Rutherford’s atomic model based on the quantum theory of radiation put forward by Max Planck.
Before going into the details of Neils Bohr model we would like to introduce to you some important atomic terms and important characteristics of waves.
ATOMIC TERMS
Nuclide : Various species of atoms in general.
Nucleons : Sub-atomic particles in the nucleus of an atom, i.e., protons and neutrons.
Isotopes : Atoms of an element with the same atomic number but different mass number.
Mass number (A) : Sum of the number of protons and neutrons, i.e., the total number of nucleons,
Atomic number (Z) : The number of protons in the nucleus of an atom. This, when subtracted from A, gives the number of neutrons.
Isobars : Atoms, having the same mass numbers but different atomic numbers, e.g.. 15P32 and 16S32.
Isotones : Atoms having the same number of neutrons but different number of protons or mass number, e.g.,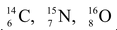 .
.
Isoelectronic : Atoms molecules or ions having the same number of electrons, e.g., N2, CO, CN-.
Nuclear isomers : Atoms with the same atomic and mass numbers but different radioactive properties, e.g., uranium X (half life 1.4 min) and uranium Z (half life 6.7 hours).
Atomic mass unit : Exactly equal to 1/12th of the mass of 6C12 atom.
(a.m.u.) : 1 a.m.u. = 1.66 × 10–24 g