
Rules For Filling Of Electrons In Various Orbitals
Atomic Structure of Class 10
The atom is built up by filling electrons in various orbitals according to the following rules.
Aufbau Principle
This principle states that the electrons are added one by one to the various orbitals in order of their increasing energy starting with the orbital of lowest energy. The increasing order of energy of various orbitals is
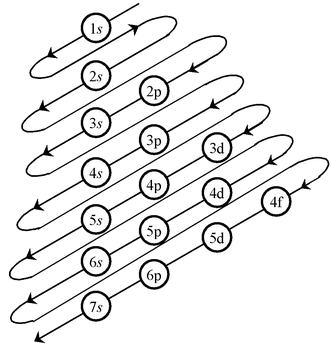
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,5f,6d,7p……………………
How to remember such a big sequence?
To make it simple we are giving you the method to write the increasing order of the orbitals. Starting from the top, the direction of the arrows gives the order of filling of orbitals.
(n +λ) Rule
Alternatively, the order of increasing energy of the various orbitals can be calculated on the basis of (n +λ) rule.
The energy of an orbital depends upon the sum of values of the principal quantum number (n) and the azimuthal quantum number (λ). This is called (n +λ) rule. According to this rule,
“In neutral isolated atom, the lower the value of (n+λ) for an orbital, lower will be its energy. However, if the two different types of orbitals have the same value of (n +λ), the orbitals with lower value of n has lower energy’’.
Illustration of (n +λ) rule
|
Type of orbitals |
Value of n |
Values of λ |
Values of (n +λ) |
Relative energy |
|
1s |
1 |
0 |
1 + 0 = 1 |
Lowest energy |
|
2s |
2 |
0 |
2 + 0 = 2 |
Higher energy than 1s orbital |
|
2p |
2 |
1 |
2 + 1 = 3 |
2p orbital (n = 2) have lower energy than 3s orbital (n = 3) |
Electronic Configuration of the Elements
ELEMENTS SYMBOL AT. NO. ELECTRONIC CONFIGURATION
Hydrogen H 1 1s1
Helium He 2 1s2
Lithium Li 3 1s2 , 2s1
Beryllium Be 4 1s2 , 2s2
Boron B 5 1s2 , 2s2 2p1
Carbon C 6 1s2 , 2s2 2p2
Nitrogen N 7 1s2 , 2s2 2p3
Oxygen O 8 1s2 , 2s2 2p4
Fluorine F 9 1s2 , 2s2 2p5
Neon Ne 10 1s2 , 2s2 2p6
Sodium Na 11 1s2 , 2s2 2p6, 3s1
Magnesium Mg 12 1s2, 2s2 2p6, 3s2
Aluminium Al 13 1s2, 2s2 2p6, 3s2 3p1
Silicon Si 14 1s2, 2s2 2p6, 3s2 3p2
Phosphorus P 15 1s2, 2s2 2p6, 3s2 3p3
Sulphur S 16 1s2, 2s2 2p6, 3s2 3p4
Chlorine Cl 17 1s2, 2s2 2p6, 3s2 3p5
Argon Ar 18 1s2, 2s2 2p6, 3s2 3p6
Potassium K 19 1s2, 2s2 2p6, 3s2 3p6, 4s1
Calcium Ca 20 1s2, 2s2 2p6, 3s2 3p6, 4s2
Scandium Sc 21 1s2, 2s2 2p6, 3s2 3p6 3d1 , 4s2
Titanium Ti 22 1s2, 2s2 2p6, 3s2 3p6 3d2 , 4s2
Vanadium V 23 1s2, 2s2 2p6, 3s2 3p6 3d3 , 4s2
Chromium Cr 24 1s2, 2s2 2p6, 3s2 3p6 3d5 , 4s1
Manganese Mn 25 1s2, 2s2 2p6, 3s2 3p6 3d5 , 4s2
Iron Fe 26 1s2, 2s2 2p6, 3s2 3p6 3d6 , 4s2
Cobalt Co 27 1s2, 2s2 2p6, 3s2 3p6 3d7 , 4s2
Nickel Ni 28 1s2, 2s2 2p6, 3s2 3p6 3d8 , 4s2
Copper Cu 29 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s1
Zinc Zn 30 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s2
Gallium Ga 31 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p1
Germanium Ge 32 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p2
Arsenic As 33 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p3
Selenium Se 34 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p4
Bromine Br 35 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p5
Krypton Kr 36 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p6
Rubidium Rb 37 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s14p65s1
Strontium Sr 38 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s1
Ytterium Y 39 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s24d1
Ziroconium Zr 40 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d2
Niobium Nb 41 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d4
Molybdnum Mo 42 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d5
Technecium Tc 43 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d5
Ruthenium Ru 44 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d7
Rhodium Rh 45 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d8
Palladium Pd 46 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d10
Silver Ag 47 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s14d10
Cadmium Cd 48 1s2, 2s2 2p6, 3s2 3p6 3d10 , 4s24p65s24d10
Indium In 49 1s2,2s2,2p6,3s2,3p6,3d10,4s24p65s24d105p1
Tin Sn 50 1s2,2s22p6, 3s2 3p6 3d10 ,4s24p65s24d105p2
Do follow NCERT Solutions for Class 10 Chemistry prepared by expert faculty of Entrancei.

