What is photoelectric effect
Atomic Structure of Class 10
Photo Electric Effect
Sir J. J. Thomson, observed that when a light of certain frequency strikes the surface of a metal, electrons are ejected from the metal. This phenomenon is known as photoelectric effect and the ejected electrons are called photoelectrons.
A few metals like Cesium, which are having low ionisation energy show this effect under the action of visible light but many more show it under the action of more energetic ultraviolet light.
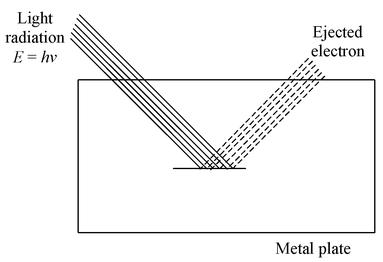
When a light radiation of sufficient energy is made to fall on the metal plate the following observations are noted.
The experimental findings are summarised as below:
- Electrons come out as soon as the light (of sufficient energy) strikes the metal surface .
- The light of any frequency will not be able to cause ejection of electrons from a metal surface. There is a minimum frequency called the threshold (or critical) frequency, which can just cause the ejection. This frequency varies with the nature of the metal. The higher the frequency of the light, the more energy the photoelectrons have.
- Photoelectric current is increased with increase in intensity of light of same frequency, if emission is permitted i.e., a bright light yields more photoelectrons than a dim one of the same frequency, but the electron energies remain the same.
Light must have stream of energy particles or quanta of energy (hν). Suppose, the threshold frequency of light required to eject electrons from a metal is νo. When a photon of light of this frequency strikes a metal it imparts its entire energy (hvo) to the electron.
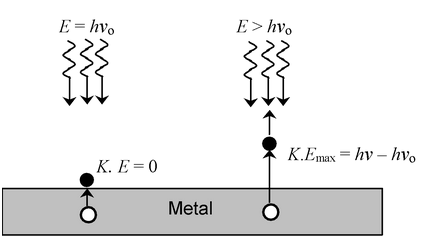
“This energy enables the electron to break away from the atom by overcoming the attractive influence of the nucleus”. Thus each photon can eject one electron. If the frequency of light is less than νo there is no ejection of electron. If the frequency of light is higher than νo (let it be ν), the photon of this light having higher energy (hν), will impart some energy to the electron that is needed to remove it away from the atom. The excess energy would give a certain velocity (i.e, kinetic energy) to the electron.
hν = hνo + K.E
hν = hνo + ½ mv2
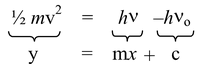
where, ν = frequency of the incident light
νo = threshold frequency, v = velocity of electron.

- Bohr's Model Of An Atom
- Rules For Filling Of Electrons In Various Orbitals
- What is photoelectric effect
- Quantum Numbers And Atomic Orbitals
- Introduction
- Dalton's Atomic Theory
- Models Of Atom
- Solved questions
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5 (True and False)
- Exercise 6 (Fill in the blanks)









