
Electrovalency
Chemical Bonding of Class 11
This type of valency involves the transfer of electrons from one atom to another, whereby each atom may attain an octet in its outermost shell. The resulting ions that are formed by the gain or loss of electrons are held together by the electrostatic force of attraction due to the opposite nature of their charges. The reaction between potassium and chlorine to form potassium chloride is an example of this type of valency.
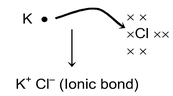
Here potassium has one electron excess of it’s octet and chlorine have one deficit of octet. So potassium donates it’s electron to chlorine forming an ionic bond.
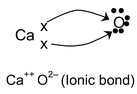
Here the oxygen accepts two electrons from a calcium atom. It may be noted that an ionic bond is not a true bond as there is no proper overlap of orbitals.
Criteria for Ionic Bond:
One of the species must have electrons over octet while the other should be in a deficit of octet. Does this mean that all substances having surplus electrons and species having deficient electrons would form an ionic bond? The answer is obviously no. Now you should ask why. The reasoning is that in an ionic bond one of the species is a cation and the other is an anion. To form a cation from neutral atom energy must be supplied to remove the electron and that energy is called ionization energy. Now it is obvious that the lower the ionization energy of the element the easier it is to remove the electron. To form the anion, an electron adds up to a neutral atom and in this process, energy is released. This process is called electron affinity.
So for an ionic bond, one of the species must have low ionization energy and the other should have high electron affinity. Low ionization energy is mainly exhibited by the alkali and alkaline earth metals and high electron affinity by the halogen and chalcogens. Therefore this group of elements is predominant in the field of ionic bonding.
