
Cell Potential And Nernst Equation
Electrochemistry of Class 12
Cell Potential And Nernst Equation
Nernst equation is used to relate either half−cell potential or emf of a cell with the concentration of the related species. Let us first consider a redox change occuring in a electrochemical cell,
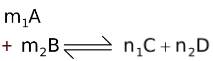
where A, B, C and D are the reagents whose concentrations varies i.e. they are either gases or in solution phase. For reagent A, the free energy per mole of A may be given thermodynamically as
GA = 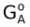 + RT ln [A]
+ RT ln [A]
For m1 moles of A, 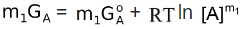
Similarly, for all other reagents,
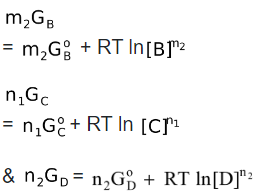
Now, the free energy change for the overall cell reaction can be deduced as
ΔG = ΔG° + RT ln 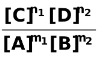 …(i)
…(i)
where ΔG° is the free energy change when all the reactants and products are present at concentrations equal to 1 M.
Any spontaneous reaction occuring in a cell, occurs with a decrease in free energy. This decrease in free energy brings in an equivalent amount of electrical work obtainable from a given system over and above any PdV energy that can be delivered to the surrounding. This can be calculated by the total charge driven through cell and the potential difference. Thus
−ΔG = Total charge × EMF of cell
−ΔG = nF × Ecell
[Negative sign indicates decrease of free energy and it implies that as Ecell becomes more and more positive, the ΔG will become more and more negative, making the reaction spontaneous]
Similarly, −ΔG° = nF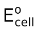
Therefore, equation (i) can be written as
−nFEcell = −nF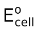 + RT ln
+ RT ln 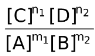
or nFEcell = 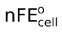 − RT ln
− RT ln 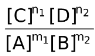
Dividing both the sides by nF gives,
Ecell = 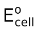 −
− 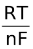 ln
ln 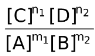
Putting T = 298 K, R = 8.314 J/mol K, F = 96500 C, we get
Ecell = 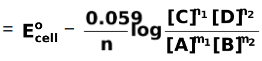 …(ii)
…(ii)
The equation (ii) is called Nernst equation, which is applicable to half cell reaction as well as to complete cell reactions.
Daniel cell represented as Zn(s)⏐ 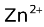 (c1)⏐⏐
(c1)⏐⏐ 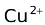 (c2)⏐Cu(s) assumes that Zn is the anode and Cu is the cathode. Such an assumption would be true only if the cell potential (Ecell) is positive. The cell potential is given in the following three ways of which we would choose the first one in all our problems.
(c2)⏐Cu(s) assumes that Zn is the anode and Cu is the cathode. Such an assumption would be true only if the cell potential (Ecell) is positive. The cell potential is given in the following three ways of which we would choose the first one in all our problems.

ERP(Cathode) is the reduction potential of the cathode while ERP(Anode) is reduction potential of the anode, EOP(Cathode) is the oxidation potential of the cathode while EOP(Anode) is the oxidation potential of the anode.
Now, let us see, how to find the EMF of Daniel cell using Nernst equation. Since we need to represent the reduction potential of cathode and anode, we first need to write the relevant reduction reactions.
For cathode: 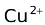 + 2e– → Cu
+ 2e– → Cu
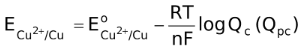
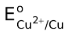 is the standard reduction potential of the given half reaction, R is the universal gas constant T is the absolute temperature at which the cell works, F is the faraday constant and n is the number of moles of electrons as seen in the reaction. The expression in the log term should be that of Kc or Kpc. This means that if reaction involves no gases, then the expression in the log term should be that of Kc while if a gas is involved then the expression in the log term should be that of Kpc. In these expressions, the concentration should be always in moles per liter while the partial pressures in atmosphere units.
is the standard reduction potential of the given half reaction, R is the universal gas constant T is the absolute temperature at which the cell works, F is the faraday constant and n is the number of moles of electrons as seen in the reaction. The expression in the log term should be that of Kc or Kpc. This means that if reaction involves no gases, then the expression in the log term should be that of Kc while if a gas is involved then the expression in the log term should be that of Kpc. In these expressions, the concentration should be always in moles per liter while the partial pressures in atmosphere units.
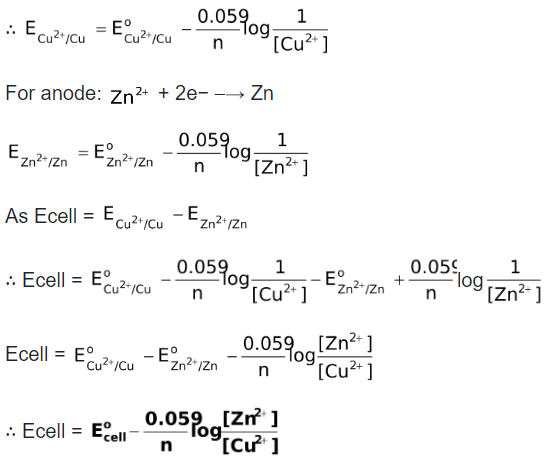
Note: Since Ecell has been defined as 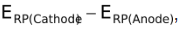 , the Nernst expression holds good even if the number of moles of electrons of the two half reactions are different.
, the Nernst expression holds good even if the number of moles of electrons of the two half reactions are different.
For example, consider the cell,
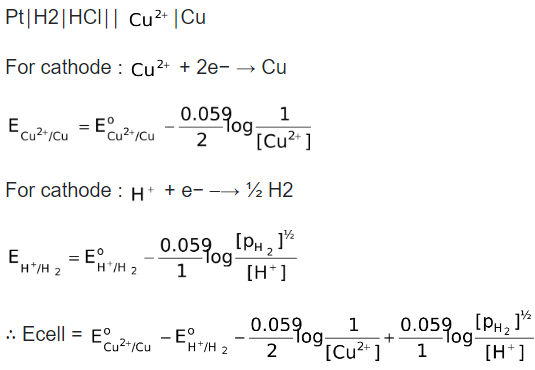
It is also possible to balance the electrons in both the half cell reactions and then subtract 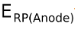 from
from . That is,
. That is,
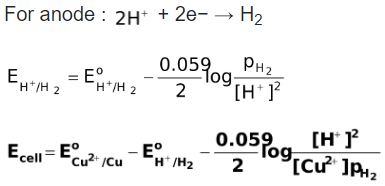
Further Reading :
