Electrolysis
Electrochemistry of Class 12
Electrolysis
|
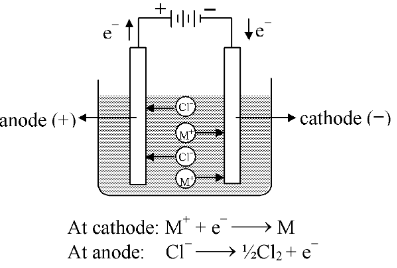
Electrolysis is a process of chemical decomposition of the electrolyte by the passage of electric current. It is carried out in a cell called electrolytic cell. This cell converts electrical energy into chemical energy. The principles of electrolytic conduction are best illustrated by reference to an electrolytic cell such as shown in figure. The entire assembly except that of the external battery is known as the electrolytic cell and the decomposition of electrolytic solutions by passing electric current is known as electrolysis. |
|
In order to pass the electric current through an electrolyte, two suitable rods of metal (or graphite) called electrodes, are inserted in the solution which are connected to a source of electromotive force (abbreviated as E. M. F.). The electrode at which the electrons enter the cell is called the negative electrode or cathode while that at which the electrons leaves is known as the positive electrode or anode. The ions which carry a positive charge (cations) move through the solution towards the cathode. Similarly, the negative ions (anion) travel towards the anode. The function of the applied emf is to direct the ions to the appropriate electrodes, and also to cause a movement of electrons from the anode to the cathode outside the cell. The flow of current is thus accompanied by removal of electrons from the anode and their transfer, through the external connecting wire to the cathode. As it can be seen below, the supply of electrons at the anode is provided by anions, while the same number of electrons at the cathode are taken by cations.
When a cation (carrying a positive charge) reaches the cathode, it gains electrons which are available at this electrode, thus having its charge neutralized. Since cations generally consist of a positively charged atoms of a metal or of hydrogen, the neutralization of the charge leaves the neutral metal or hydrogen deposited upon the cathode. This result is in accordance with the statement made above that the passage of an electric current through an electrolyte is usually associated with the visible separation of matter. Similarly when an anion (negatively charged) reaches the anode, the electrons are removed, leaving the discharged neutral atom or group of atoms. If the anion is a halogen or hydroxyl ion, the discharged material may appear as the free halogen or as oxygen, respectively. Some anions such as sulphate, nitrate and phosphate are usually not discharged from aqueous solutions, and so other processes, accompanied by the loss of electrons take place at the anode. One of these is the liberation of oxygen from water. Another is the abstraction of electrons from the atoms of the actual metal constituting the anode. The atoms are thus converted into the corresponding positively charged cations which pass into the solution. Such attackable metals as copper, zinc etc. usually dissolve when they are used as anodes. On the other hand, platinum and gold fall into the category of "unattackable metals" that does not pass into the solution at all, when they are used as anodes.
Further Reading :
1. Qualitative Aspect of Electrolysis