
Concentration Cells
Electrochemistry of Class 12
Concentration cells are those cells whose 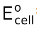 is zero.
is zero.
Concentration cells are of two types,
- Electrode Concentration Cells
- Electrolyte Concentration Cells
Electrode Concentration Cells
In these cells, two similar electrodes at different concentrations are dipping in the same solution. Two hydrogen electrodes at unequal gas pressures immersed in the same solution of hydrogen ions constitute an electrode concentration cell. This may be represented as follows
Pt ⏐H2(p1) | solution of H+ ions |H2(p2)⏐ Pt
The reactions occurring are
At cathode: 2H+ + 2e– → H2(p2)(Reduction)
At anode:H2(p1) → 2H+ + 2e–(Oxidation)
Net reaction:H2(p1) → H2(p2)
This reaction is evidently independent of the concentration of the electrolyte.
At moderate pressures, H2 can be considered to be an ideal gas so that the ratio of the fugacities can be considered to be equal to the ratio of the gas pressures. Hence, the Nernst equation may be written as
At 25 °C
Since, by definition, 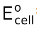 = 0, we have
= 0, we have
Ecell = –0.0295 log 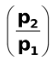 = 0.0295 log
= 0.0295 log 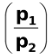
When p2 <p1, the EMF is positive so that the whole process is spontaneous.
Another example of the electrode concentration cell is that of an amalgam with two different concentrations of the same metal
Hg–Pb(c1)⏐PbSO4 (soln.)⏐Hg–Pb(c2)
The electrode reactions are
At cathode: Pb2+ + 2e– → Pb(c2)(Reduction)
At anode:Pb(c1) → Pb2+ + 2e–(Oxidation)
Net reaction:Pb(c1) →Pb(c2)
The EMF of the cell is given by
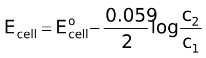
Here, too, if c2 < c1, the EMF is positive so that the whole process is spontaneous, i.e., lead will go spontaneously from the high concentration to the low concentration amalgam.
Electrolyte Concentration Cells
In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different concentrations. One such cell is represented as
Zn⏐ Zn2+ (c1)|| Zn2+(c2)⏐Zn
In this case, both the electrodes are of the same metal (Zn) and these are in contact with solutions of the their metal ions ( Zn2+). The concentrations and hence activities of the ions are however, different. Let (c1) and (c2) be the concentrations of zinc ions in the two electrolyte (ZnSO4) solutions which are separated from each other by a salt bridge.
The electrode reactions are
At cathode: Zn2+ (c2) + 2e− → Zn(s) (Reduction)
At anode:Zn(s) → Zn2+(c1) + 2e– (Oxidation)
Net reaction: Zn2+(c2) → Zn2+(c1)
According to Nernst equation, the reduction potentials of cathode and anode are given by
For the process to be feasible (spontanoues), EMF should be positive. Hence, c2 > c1.
