
Different Types Of Voltaic Cells
Electrochemistry of Class 12
Different Types Of Voltaic Cells
There are two types of voltaic cells :
(1) Primary (2) Secondary
Primary Cells
A cell which acts as a source of electricity without being previously charged by an electrical current from an external source is called primary cell. In such a cell electrical energy is obtained at the expense of chemical reactivity only as long as the active materials are present. Dry cell is an example of primary cell.
Examples of Primary Cells
(1) Dry Cell
The most commonly used flashlight battery is the dry cell, also called the Leclanche cell, after its inventor, Georges Leclanche. The anode of a dry cell is a zinc can (or cup), which is usually covered with a steel jacket to shield it from the atmosphere. The cathode is a graphite rod which serves as an inert electrode. The graphite rod is in the center of the cell and is surrounded by a thick paste containing MnO2 and powdered graphite. It is the MnO2 that is reduced at the cathode. The electrolyte is a moist paste of a saturated solution of NH4Cl, ZnCl2 and some inert filler. The cell is not really dry, water is an essential component of the electrolytic paste.
At the anode, Zn is oxidized to the +2 state, and at the cathode MnO2 is reduced to the +3 state. There are a number of different chemical substances formed involving the +3 oxidation state of manganese, including Mn2O3(s), , and MnO(OH). We will write the cell reactions showing the formation of Mn2O3 only at the cathode.
At anode: Zn(s) 
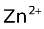 + 2e−
+ 2e−
At cathode: 2MnO2(s) +  + 2e−
+ 2e−  Mn2O3(s) + 2NH3 + H2O
Mn2O3(s) + 2NH3 + H2O
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
Net reaction: Zn(s) + 2MnO2(s) + 
 Mn2O3(s) +
Mn2O3(s) + 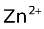 + 2NH3 + H2O
+ 2NH3 + H2O
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
The voltage of a dry cell battery is 1.5 V.
The dry cell is not rechargeable, as the 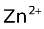 ions formed at the anode migrate through the electrolytic paste and combine with the NH3 produced at the cathode to form the complex
ions formed at the anode migrate through the electrolytic paste and combine with the NH3 produced at the cathode to form the complex
ion, .
One of the problems with the dry cell is that the electrolytic paste is acidic, since NH4Cl is an acidic salt. Thus there is a direct reaction between Zn and that slowly eats away the zinc can.
Zn(s) + 

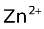 + H2(g) + 2NH3
+ H2(g) + 2NH3
A flashlight battery that has never been used but has been sitting on the shelf for more than a year may split and leak as the Zn metal is converted to 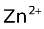 ions by above reaction.
ions by above reaction.
An improved form of the dry cell is the alkaline dry cell, in which the NH4Cl is replaced by
KOH. It is more expensive than the acid form, but it lasts longer because there is no corrosion of the Zn by ions.
(2) Fuel Cell
At Anode : H2 + → 2H2O(l) + 2e−
At Cathode : O2 + 2H2O + 4e− → 4OH− (aq)
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
Net reaction : 2H2(g) + O2(g) → 2H2O(l)
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
Secondary Cells
A secondary cell once used can be recharged by passing current through it and it may, therefore, be used over and over again. Certain chemical changes occur when the cell is charged with electricity and these changes are reversed during discharge.
Examples of Secondary Cells
Lead Storage Battery
The battery used in all automobiles is a lead storage battery. It is a reversible cell, acting as electrochemical cell when discharged and during charging process, it acts as electrolytic cell. The anode and cathode reactions get reversed during charging and discharging. The anode (negative terminal) consists of a lead electrode. The substance reduced at the cathode is lead dioxide, PbO2. Since PbO2 is a powdery solid, the cathode is made of a lead grid with the interstices filled with PbO2. The anode is also a lead grid, with the interstices filled with spongy lead for greater reactivity. Both electrodes are immersed in the same aqueous solution of sulfuric acid, 35% H2SO4 by weight.
Pb ⏐ PbSO4(s) ⏐ H2SO4(aq) ⏐ PbO2 (s) | Pb
At the anode, lead is oxidized from the 0 state to the +2 state, and the insoluble white salt of PbSO4 precipitates out. At the cathode, lead is reduced from the +4 state in PbO2 to the +2 state, and PbSO4 again precipitates out. As the cell is used, the interstices of both grids get filled with PbSO4. The two half−reactions during discharging are
At cathode: PbO2(s) + + + 2e−  PbSO4(s) + 2H2O
PbSO4(s) + 2H2O
At anode: Pb(s) + 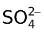
 PbSO4(s) + 2e−
PbSO4(s) + 2e−
____________________________________________________
Net cell reaction: Pb(s) + PbO2(s) + 2H2SO4  2PbSO4(s) + 2H2O
2PbSO4(s) + 2H2O
One of the conveniences of this battery is that no salt bridge or porous barrier is needed, both electrodes are immersed in the same solution. The short hand notation for this battery therefore does not have a double vertical bar, and appears as follows
Pb(s) | PbSO4(s) | H2SO4 (35% aq soln) | PbSO4(s) | PbO2(s) | Pb(s)
One cell of a lead storage battery has a potential of about 2.0 V. It is common practice to construct a battery by combining six such cells in series to provide 12.0 V.
As the battery is used, the solution in the cell becomes more dilute, since H2O is a product of the net cell reaction and H2SO4 is used up during the reaction. The density of the electrolytic solution therefore decreases as the battery discharges, and a measurement of the density can be used as a simple way to tell just how far the cell has discharged. This is important because a lead storage battery has an extremely advantageous feature that it can be recharged. The PbSO4 formed when the cell is discharged remains embedded in the interstices of the grids of the electrodes, so that if electricity is passed into the cell by using some external source of energy, the cell reaction can be reversed. The PbSO4 is reconverted into Pb at one electrode and PbO2 at the other.
The cell reaction is easily reversed if the PbSO4 is freshly precipitated, but as PbSO4 stands it changes its crystalline structure and ages to a much less reactive form. A battery that has been discharged for a few weeks or more cannot be fully recharged. Storage batteries in gasoline powered automobiles are recharged as you drive, so that they never become fully discharged unless there is some malfunction.
