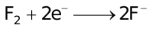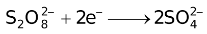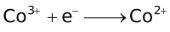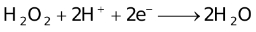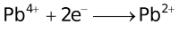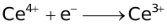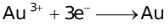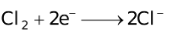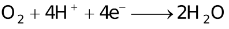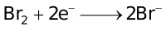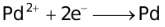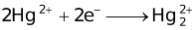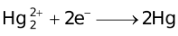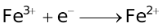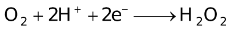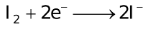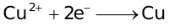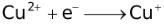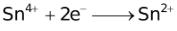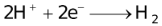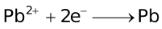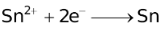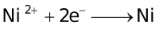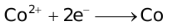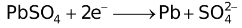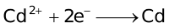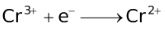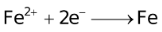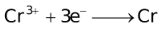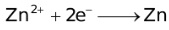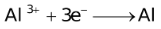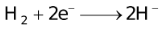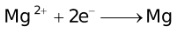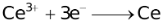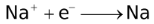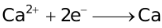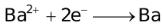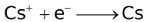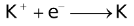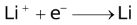Qualitative Aspects Of Electrolysis
Electrochemistry of Class 12
Qualitative Aspects Of Electrolysis
Now let us assume a situation when an electrolyte contains a number of different cations then, provided there are no disturbing factors, each cationic discharge will take place as soon as the appropriate potential is reached.
The ability of a cation to move towards the negative electrode and get reduced depends upon the size, mass, positive charge, mobility etc. of the ion. It is thus not possible to predict qualitatively the order of reduction of cations, as one factor might enhance it while the other factor might hamper it. The only way we can predict this, is by giving a quantitative value based on the cumulative effect of all the factors responsible for a cation's ability to get reduced. This quantitative value is called the standard reduction potential (SRP). The SRP values at 25 °C are given below for some reactions.
Standard Reduction Potentials at 25 °C
|
S.No. |
Half reaction |
E° in volts |
|
1. |
|
+2.652. |
|
2. |
|
+2.01 |
|
3. |
|
+1.82 |
|
4. |
|
+1.77 |
|
5. |
|
+1.70 |
|
6. |
|
+1.61 |
|
7. |
|
+1.50 |
|
8. |
|
+1.36 |
|
9. |
|
+1.229 |
|
10.
|
|
+1.07 |
|
11. |
|
+0.92 |
|
12. |
|
+0.92 |
|
13. |
|
+0.799 |
|
14. |
|
+0.79 |
|
15. |
|
+0.77 |
|
16. |
|
+0.69 |
|
16. |
|
+0.535 |
|
17. |
|
+0.34 |
|
18. |
|
+0.27 |
|
19. |
|
+0.222 |
|
20. |
|
+0.15 |
|
21. |
|
+0.13 |
|
22. |
|
0.00 |
|
23. |
|
−0.126 |
|
24. |
|
−0.14 |
|
25. |
|
−0.151 |
|
26. |
|
−0.17 |
|
27. |
|
−0.25 |
|
28. |
|
−0.28 |
|
29. |
|
−0.31 |
|
30. |
|
−0.403 |
|
31. |
|
−0.41 |
|
32. |
|
−0.44 |
|
33. |
|
−0.74 |
|
34. |
|
−0.762 |
|
35. |
|
−0.828 |
|
36. |
|
−1.66 |
|
37. |
|
−2.25 |
|
38. |
|
−2.37 |
|
39. |
|
−2.48 |
|
40. |
|
−2.71 |
|
41. |
|
−2.87 |
|
42. |
|
−2.90 |
|
43. |
|
−2.92 |
|
44. |
|
−2.93 |
|
45. |
|
−3.03 |
When the external EMF applied to an electrolytic cell (containing more than one cation) is gradually increased, the potentials of the electrodes change until the reduction potential of the most easily discharged cation is attained. The cations that are discharged most readily are those having the largest reduction potential, since the free energy of the process 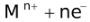 → M will then have its greatest negative value, indicating a considerable tendency for the discharge process to occur. Thereafter, if the electrolysis is prolonged to such an extent that the cation which gets discharged first, gets almost exhausted, then another cation from the solution gets discharged (obviously this cation would have lesser reduction potential than the first cation).
→ M will then have its greatest negative value, indicating a considerable tendency for the discharge process to occur. Thereafter, if the electrolysis is prolonged to such an extent that the cation which gets discharged first, gets almost exhausted, then another cation from the solution gets discharged (obviously this cation would have lesser reduction potential than the first cation).
Sometimes simultaneous deposition of two metals occur in the form of an alloy. For instance, let us take copper and zinc in a solution containing their cyanide complex. Although the potentials of these metals differ considerably in sulphate solutions, the ionic concentrations are so changed in the complex cyanides solutions so as to bring their respective potentials close together. When this solution is electrolyzed, an alloy of Zn and Cu i.e., brass is deposited on the cathode.
It should also be pointed out that all aqueous solutions contain hydrogen ions which consequently discharges with the liberation of H2. The discharge of certain cations such as tin, nickel, iron etc. is almost invariably accompanied by the evolution of H2.
[Note: It must be remembered that it is not the SRP of a cation that determines its discharge, this in turn is decided by its reduction potential. The SRP's must be used only when the concentration of ions are given as 1 M. For concentrations other than 1 M, the reduction potentials for each cation at that concentration is to be calculated and then the discharge of an ion is predicted. The relation between the reduction potential and standard reduction potential is given by Nernst equation which is as follows:
ERP = 
where ERP = Reduction potential of ion and
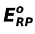 = Standard reduction potential of ion]
= Standard reduction potential of ion]
In general, the behaviour at an anode is analogous to that at cathode. The process associated with the largest negative free energy change (largest oxidation potential), whether it be solution of the metallic anode to form cations or the discharge of anions, will take place first. Subsequent anodic processes will follow in the order of decreasing oxidation potentials.
For example, if a copper electrode is placed in an acid solution of 1 M CuSO4, then three anodic processes are possible. Firstly, Cu goes into the solution as Cu2+ ions. Secondly, hydroxyl ions (which are always present in aqueous solution) may discharge and thirdly, 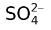 may get discharged. From the table of SRP, it is evident that when an external emf is applied to the copper anodes, the first process to occur will be that of the anode passing into the solution as cupric ions. The next possible process is the discharge of hydroxyl ions, but this will not occur unless for some reason the solution of the anode is prevented. If an unattackable anode such as Pt is used, hydroxyl ions would be discharged and oxygen is evolved, as anion discharge is the possible process that can occur at anode. The discharge of sulphate ions in any event is highly improbable.
may get discharged. From the table of SRP, it is evident that when an external emf is applied to the copper anodes, the first process to occur will be that of the anode passing into the solution as cupric ions. The next possible process is the discharge of hydroxyl ions, but this will not occur unless for some reason the solution of the anode is prevented. If an unattackable anode such as Pt is used, hydroxyl ions would be discharged and oxygen is evolved, as anion discharge is the possible process that can occur at anode. The discharge of sulphate ions in any event is highly improbable.
[Note: The discharge of 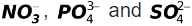 ions at anode does not commonly take place from aqueous solutions.]
ions at anode does not commonly take place from aqueous solutions.]