
Kohlrausch’s Law Of Independent Migration Of Ions
Electrochemistry of Class 12
Kohlrausch’s Law Of Independent Migration Of Ions
While investigating the equivalent conductances at infinite dilution (λo), Kohlrausch found that when salts of potassium and sodium with a common anion are taken, the difference in their λo−values was found to be same irrespective of the nature of the anion. That is etc. Similar results were also obtained with other pairs of salts having either a common anion or a common cation. A few of the results are shown below.
Table: λo−Values for pairs of electrolytes (25°C)
|
Electrolyte |
λo |
Difference λo(1) − λo(2) |
Electrolyte |
λo |
Difference λo(1) − λo(2) |
|
(1) KCl |
149.9 |
(1) LiCl |
115.0 |
||
|
(2) NaCl |
128.8 |
21.1 |
(2) LiNO2 |
110.1 |
4.9 |
|
(1) KNO3 |
145.0 |
(1) KCl |
149.9 |
||
|
(2) NaNO3 |
123.9 |
21.1 |
(2) KNO3 |
145.0 |
4.9 |
|
(1) KNO3 |
145.0 |
(1) HCl |
426.16 |
||
|
(2) LiNO3 |
110.1 |
34.9 |
(2) HNO3 |
421.3 |
4.9 |
|
(1) KClO4 |
140.0 |
(1) NaCl |
128.8 |
||
|
(2) LiClO4 |
105.08 |
34.92 |
(2) NaNO3 |
123.9 |
4.9 |
|
(1) KCl |
149.9 |
|
|
(2) LiCl |
115.0 |
34.9 |
Such constancy in the difference of λo−values of two salts having a common ion is possible only if each ion makes a definite contribution towards the conductivity of the solution irrespective of the value of the other ion. Thus,
Hence, whatever salts of sodium and potassium be taken, provided the salts are taken at infinite dilution with common anion the difference in λo value will be always same.
Kohlrausch therefore proposed that at infinite dilution, each ion makes a definite contribution towards the equivalent conductance of the electrolyte. This contribution is independent of the other ion with which it is present in the solution. The value of the equivalent conductance at infinite dilution (λo) of an electrolyte is the sum of contributions of the two ions, such that
λo = …(iv)
where and are the contributions of the cation and anion respectively, called ‘ion conductances’.
That is, at infinite dilution, the equivalent conductance is the sum of ion conductances, which are characteristic of each ion species. This is commonly known a the law of independent migration of ions.
The individual ionic conductances are usually determined from their transport number at infinite dilutions. At infinite dilution, an electrolyte is completely dissociated and there is no interionic attraction. In conveying a current, the conduction due to the cations would depend upon their charge and their speed. In other words, ion conductance lc will depend upon charge (n+ z+ e) carried to the cathode and the speed u.
or = k1n+ z+ eu
= k u
Similarly for the anions, = k1n− z− ev
∴ From equation, (iv)
λo = + = k1n+ z+ eu + k1n− z− ev
Now, the net charges released at the cathode and anode being equal
∴ k1z+ n+ e = k1 z− n− e
∴ λo = k (u + v)
where k = k1 z+ n+ e
= k1 z− n− e
∴
Hence from equation, (v) i.e. and
The zero−subscripts are used to indicate the state of infinite dilution in the application of these relations.
The transport numbers measured at low concentrations of an electrolyte are extrapolated to zero concentration to obtain and . The knowledge of the transport numbers (to) together with a determination of λo would enable us to obtain the values of ion conductances (lo).
(Application: In the case of weak electrolytes λo, the conductance at infinite dilution cannot be directly obtained. In fact, the law of independent migration of ions is used for finding out λo for weak electrolytes. To illustrate: λo for acetic acid is evaluated by determining the equivalent conductance of three strong electrolytes separately, namely, HCl, NaCl and NaAc. It is easily seen.
Since the quantities on the left−hand side are experimentally determined, λo is known. This method is employed for other weak electrolytes also).
Example 6
By how much is the oxidising power of the couple decreases if the H+ concentration is decreased from 1 M to 10−4 M at 25°C. Assume other species have no change in concentration.
Solution
In acidic medium, acts as oxidising agent as per the reaction
+ 8H+ + 5e− Mn2+ + 4H2O
∴ (E1 − E2) = = 0.3776 V
Thus, the oxidising power of couple decreases by 0.3776 V from its standard value.
Example 7
EMF of the following cell is 0.67 V at 298 K.
Pt | H2 (1 atm) | H+ (pH = X) || KCl (1 N) | Hg2Cl2(s) | Hg
Calculate the pH of anode compartment. Given = 0.28 V
Solution
At anode: H2 2H+ + 2e−
At cathode: Hg2Cl2 + 2e− 2Hg + 2Cl−
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
Net reaction: H2 + Hg2Cl2 2H+ + 2Hg + 2Cl−
∴Ecell = −−
0.67 = 0.28 − = 0.28 − 0.059 log [H+]
0.67 = 0.28 + 0.059 pH
∴pH = 6.61
Example 8
The standard reduction potential of the Ag+ | Ag electrode at 298 K is 0.799 V. K</spansp of AgI is 8.7 × 10−17. Evaluate the potential of the Ag+ | Ag electrode in a saturated solution of AgI. Also calculate the standard reduction potential of I− | AgI | Ag electrode.
Solution
Ag+ + e− Ag
= 0.799 − 0.059 log = 0.325 V
AgI 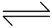 Ag+ + I− …(i)
Ag+ + I− …(i)
Ag+ + e− Ag …(ii)
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
AgI + e− Ag + I− …(iii)
We have learnt that metal−insoluble salt−anion electrode and metal−metal ion electrode has same potentials. Thus, equation (ii) and (iii) would have same reduction potential.
∴
∴ 0.325 = − 0.059 log = − 0.059 log
∴ = −0.149 V
Example 9
Calculate the EMF of the electrode-concentration cell
Zn–Hg(c1) | Zn2+(aq) | Zn–Hg(c2)
at 25 °C, if the concentrations of the zinc amalgam are: c</span1 = 2 g per 100 g of mercury and c</span2 = 1 g per 100 g of mercury.
Solution
The cell reactions in this case are
At cathode: Zn2+ + 2e– Zn(c2) (Reduction)
At anode: Zn(c1) Zn2+ + 2e– (Oxidation)
Net reaction: Zn(c1) Zn(c2)
∴ = 8.8 × 10–3 V
Example 10
Calculate the EMF of the concentration cell consisting of zinc electrodes, one immersed in a solution of 0.01 M Zn2+ ions and the other in a solution of 0.1 M Zn2+ ions at 25 °C. The two solutions are separated by a salt bridge.
Solution
The cell may be represented as
Zn | Zn2+ (0.01 M)⏐⏐Zn2+ (0.1 M) | Zn
The EMF of the cell is given by
= = 0.0295 V
Example 11
Calculate emf of the cell
Pt | H2 | CH3COOH (0.1 M) || NH4OH (0.01 M) | H2 | Pt
Ka for CH3COOH = 1.8 × 10−5 and Kb for NH4OH = 1.8 × 10−5
Solution
For CH3COOH
CH3COOH CH3COO + H+
∴ Ka =
or Ka = or [H+] =
∴ [H+] = 1.3461 × 103 M
For NH4OH
NH4OH + OH−
Similarly
∴ [OH−] =
= 4.2426 × 104 M
∴ [H+] = = 2.357 × 10−11
Cell reaction
At anode H2 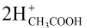 + 2e−
+ 2e−
At cathode + 2e− H2
———————————————
On applying Nernst equation
Ecell = E°cell −log
Ecell = 0 −log
= 0.0591 log = 0. 4575 V
Example 12
Consider the reaction, 2Ag+ + Cd → 2Ag + Cd 2+. The standard electrode potentials for Ag+/Ag and Cd2+/Cd couples 0.80 V and 0.40 V respectively. (i) What is the standard potential for this reaction? (ii) For the electrochemical cell in which the above reaction takes place which electrode is negative electrode ? (iii) Will the total emf of the given reaction be more negative or positive, if the concentration of Cd2+ ions is 0.1 M rather than 1.0 M ?
Solution
(i) In the given reaction Cd is being oxidised to Cd2+ ions and Ag+ ions are being reduced to Ag metal. Thus, the galvanic cell in which the given reaction takes place can be represented as
Cd(s) | Cd2+(aq) || Ag+(aq) | Ag(s)
Thus, the standard potential of the given reaction would be the standard emf of the above cell (E°cell) which is given by
E°cell = E°Cathode − E°Anode
= 0.80 − 0.40 = 0.40 V
(ii) Cd(s)|Cd2+(aq) electrode constitutes the negative electrode of the cell, since it is the electrode at which oxidation takes place.
(iii) The cell in which the concentration of Cd2+ ions is 0.1 M and not 1.0 M can
be shown as
Cd(s) | Cd2+(0.1M) || Ag+ (1.0 M) | Ag(s)
Cell reaction Cd(s) + 2Ag+ 2Ag(s) + Cd2+(aq)
For this cell, Ecell = E°cell −log
= 0.40 − 0.0295 × log
= 0.40 − 0.0295 × log 10−1
= 0.40 + 0.0295 × 1 = 0.4295 V
Example 13
A zinc rod is placed in 0.1 M solution of ZnSO4 at 25°C. Assuming that the salt is dissociated to the extent of 95% at this dilution, calculate the potential of the electrode at this temperature. Given that = − 0.76 volts.
Solution
Concentration of ZnSO4 solution = 0.1 M
Percentage of dissociation of ZnSO4 solution = 95%
Concentration of Zn2+ ions in ZnSO4 solution = = 0.095 M
Thus, the electrode can be represented as
Zn(s) | Zn2+(0.095 M)
Reduction reaction taking place at this electrode is
Zn2+ + 2e− → Zn(s) (Here n =2)
According to the Nernst equation, the reduction potential of the above electrode is given by −log
or −0.76 + log 0.095
or −0.79 volt
Example 14
The emf of the cell
Zn | ZnCl2(0.05 mol dm−3) | Cl– | AgCl(s) | Ag
is 1.015 V at 298 K, the silver electrode being positive, while the temperature coefficient of its emf is −0.000492 VK−1. Write down the equation for the reaction occuring when the cell is allowed to discharge and calculate the changes in (a) free energy (G), and (b) heat content (H) and (c) entropy (S) accompanying this reaction, at 298 K.
Solution
Cell reaction
At anode:
Zn → Zn2+ + 2e−
At cathode: 2AgCl(s) + 2e− → 2Ag+ + 2Cl−
—————————————
Zn + 2AgCl(s) → 2Ag + Zn2+ + 2Cl−
(a) The free energy change accompanying the reaction is given by
ΔG = −nFE
n =2, E = 1.015 V, F = 96500 C mol−1
ΔG298 = −2 × 1.015 V × 96500 C mol−1
ΔG298 = −195895 J mol−1
= −195.895 kJ mol1
(b) ΔH = − nFE + nFT
∴ ΔH298 = −195.895 kJ mol−1 + ΔH298 = −224.20 kJ mol–1
(c) ΔG = ΔH − T ΔS
ΔS =
ΔS298 = nF
= (−2 × 96500 × 0.000492) JK1 mol1
= −95.0 J K−1 mol−1
Example 15
For the following galvanic cell, calculate the emf at 25°C. Assign the correct polarity for the spontaneous reaction to take place
Ag | AgCl(s), KCl (0.2 M) || KBr (0.001 M), AgBr(s) | Ag
The solubility product of AgCl and AgBr are 2.8 × 10−10 and 3.3 × 10−13 respectively.
Solution
On the basis of the cell as given we have anode as:
Anode: Ag(s) Ag+(c1) + e− (Oxidation)
At cathode : Ag+(c2) + e− Ag(s) (Reduction)
For concentration cell
Ag | AgCl(s), KCl (0.2 M) || KBr (0.001M), AgBr(s) | Ag
Net reaction is
Ag+ (c2) Ag+(c1)
For this reaction in above concentration cell
ECell = log10 ….(i)
AgCl Ag+ + Cl−
∴ Ksp of AgCl = [Ag+] [Cl−]
Ksp of AgCl = 2.8 × 10−10 [Cl−] of KCl = 0.2 M
(Because KCl is assumed as 100% ionised)
∴ 2.8 × 10−10 = [Ag+] × 0.2
∴ [Ag+] == 14 × 10−10 M
[Ag+] of AgCl = C1
∴ C1 = 14 × 1010 M
Now AgBr Ag+ + Br
Ksp of AgBr = [Ag+] [Br−]
Ksp of AgBr = 3.3 × 10−13
(Because KBr is also assumed as 100 % ionised)
∴ 3.3 × 10−13 = [Ag+] [0.001]
∴ [Ag+] = = 3.3 × 10−10 M
This [Ag+] of AgBr = C2
∴ C2 = 3.3 × 10−10 M
Substituting the values of C1, C2 and n (n =1) in equation (i), we have
Ecell = 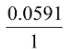 log = −0.337 V
log = −0.337 V
Since Ecell has negative value, the cell will not function as it is given. Thus, the polarities will be reversed for the spontaneous reaction. The cell will be constructed as follows.
Ag | AgBr(s) || AgCl(s) | Ag
Example 16
The standard reduction potential of Cu2+/Cu and Ag+/Ag electrodes are 0.337 and 0.799 volt respectively. Construct a galvanic cell using these electrodes so that its standard emf is positive. For what concentration of Ag+ will the emf of the cell at 25°C be zero, if the concentration of Cu2+ is 0.10 M ?
Solution
Since = 0.799 V is greater than = 0.337 V. Ag+(aq)/Ag(s) will form the cathode and Cu(s)/Cu+2 (aq) will behave as anode of the galvanic cell. Thus, the cell can be represented as given below. Here M1 is the concentration of Ag+(aq) ions
Cu(s) | Cu2+( 0.01M) || Ag+(c) | Ag(s)
Cell reaction is Cu(s) + 2Ag+(aq)  Cu2+(aq) + 2Ag(s)
Cu2+(aq) + 2Ag(s)
Here n =2
From Nernst equation at 298 K, we have
Ecell = E°cell − log
= [ − ] −0.0295 log
At equilibrium Ecell =0
0.0 = (0.799 − 0.337) − 0.0295 log
0.462 = 0.0295 log
or log = = 15.6610
or = antilog 15.6610 = 4.571 × 1015
∴ c2 =
Hence
∴ [Ag+] = 1.47 × 109 M.
