Abnormal Molecular Weight And Vant Hoff Factor
Liquid Solution of Class 12
Abnormal Molecular Weight And Vant Hoff Factor
Since colligative properties depend upon the number of particles of the solute, in some cases where the solute associates or dissociates in solution, abnormal results for molecular masses are obtained.
Van't Hoff Factor: Van't Hoff, in order to account for all abnormal cases introduced a factor i known as the Van't Hoff factor, such that

i > 1 for dissociation of solute in solution
i < 1 for association of solute.
i = 1 when solute neither associate non dissociate.
Some other properties of liquids
Many familiar and observable properties of liquids can be explained by the intermolecular forces. Some liquids such as water or gasoline flow easily when poured, whereas other such as motor oil or maple syrup, flow sluggishly.
The measure of a liquids resistance to flow is called its viscosity. Viscosity is related to ease with which individual molecules move around in the liquid and thus to the intermolecular forces present. Substance with small nonpolar molecules, such as pentane and benzene, experience only weak intermolecular forces and have relatively low viscosities whereas more polar substances such as glycerol (C3H5(OH)3) experience stronger intermolecular forces and so have higher viscosities.
Another familiar property of liquids is surface tension, the resistance of a liquid to spread out and increase its surface area. Surface tension is caused by the difference in intermolecular forces experienced by molecules at the surface of a liquid and those experienced by molecules in the interior. Molecules at the surface feel attractive forces on single side only and are thus drawn in toward the liquid, white molecules in the interior are surrounded and are drawn equally in all directions. Surface tension, like viscosity is generally higher in liquids that have stronger intermolecular forces. Both properties are also temperature dependent because molecules have more kinetic energy to counteract the attractive forces holding them together.
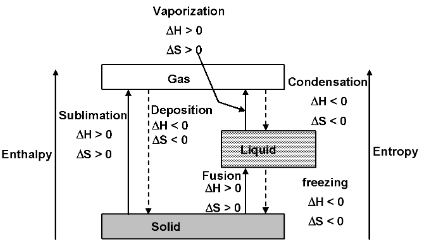
Phase changes
1. Fusion (melting) Solid → Liquid
2. Freezing Liquid → Solid
3. Vaporization Liquid → Gas/vapour
4. Condensation Gas → liquid
5. Sublimation Solid → Gas
6. Deposition Gas → Solid
Every phase change has associated with it a free energy change, ΔG. This free energy change ΔG is made up of two contributions on enthalpy part ΔH and a temperature – dependent entropy part TΔS, according to equation 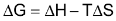
The enthalpy part is the heat flow associated with making or breaking the intermolecular attractions that hold liquids and solids together, while the entropy part is associated with the change in disorder or randomness between the various phases.
|
|
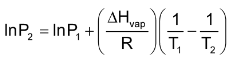
The Clausius-clapeyron equation
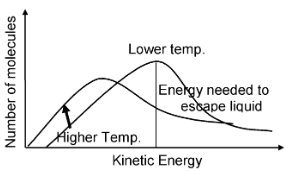
The molecules in a liquid are in constant motion but at a variety of speeds depending on the amount of energy (K.E.) they have. The higher the temperature and the lower the boiling point of the substance, the greater the fraction of molecules in the sample that have sufficient kinetic energy to break free from the surface of the liquid and escape into the vapour.
|
|
Only the faster moving molecules have sufficient kinetic energy to escape from the liquid and enter the vapour.
The vapour pressure of a liquid rises with temperature in a non linear way. A linear relationship is found, however, when the logarithm of the vapour pressure, in vapour is plotted against the inverse of the Kelvin temperature, 1/T.
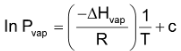
y = m, x + c
Where ΔHvap is the heat of vaporization of liquid, R is the gas constant and c is a constant characteristic of each specific substance.
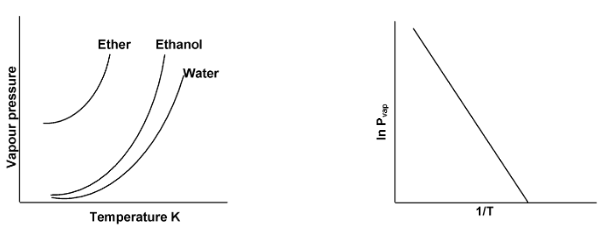
The clauses clapeyron equation makes it possible to calculate the heat of vaporization of a liquid by measuring its vapour pressure at several temperature and than plotting to obtain the slope of the line
It can be written in this way too.