
Ideal Solutions
Liquid Solution of Class 12
Ideal Solutions
An ideal solution of substance A and B is one in which both substances follow Raoult’s law for all values of mole fractions. Such solutions occurs when the substances are chemically similar so that the intermolecular forces between A and B molecules are similar to those between two A molecules or between two B molecules. The total vapour pressure over an ideal solution equals the sum of the partial vapour pressures, each of which is given by Raoult’s law.
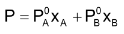
For example: Solution of benzene C6H6 and toluene C6H5CH3 are ideal. Note the similarly in their structural formula.
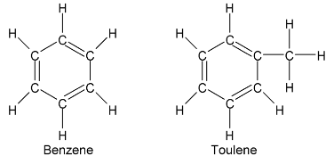
Suppose a solution is 0.70 mole fraction benzene and 0.30 mole fraction of toluene. The vapour pressure of pure benzene and pure toluene are 75 mmHg and 22 mmHg respectively. Hence the total vapour pressure is
P = 75 x 0.70 + 22 x 0.30 = 59 mmHg
Ethylene bromide and ethylene chloride, n-Hexane and n-heptane, n-butyl chloride and
n – butyl bromide, carbon tetrachloride and silicon tetra chloride are few other examples of ideal solutions.
Condition for a solution to be ideal.
(a) It should obey Raoult’s law i.e. 
(b) 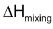 = 0 i.e. no heat should be absorbed or evolved during mixing.
= 0 i.e. no heat should be absorbed or evolved during mixing.
(c)  = 0 i.e. no expansion or contraction on mixing
= 0 i.e. no expansion or contraction on mixing
REAL OR NON - IDEAL SOLUTIONS
Those solution which do not obey Raoult’s law over entire range of composition and deviate from ideal behaviour, are real or non – ideal solution. They are divided into two types
(a) The solution showing positive deviations from ideal behaviour for those type of solutions,
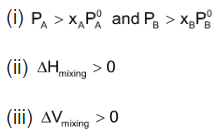
Positive deviation
Solution of ethyl alcohol in water shows positive deviation. Attractive forces between
A – A or B − B > B − B.
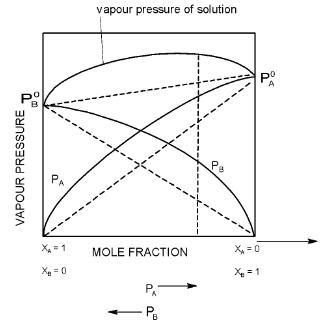
Positive deviations occur when the interaction between unlike molecules is weaker than the interaction between like molecules. The solutions of this type are characterized by positive enthalpy of mixing, ΔHmix and positive volume of mixing, ΔVmix. Example are ⎯acetaldehyde ⎯carbon disulphide, water ⎯propyl alcohol, ethyl alcohol ⎯chloroform and cyclohexane ⎯carbon tetrachloride.
(b) The solution showing large negative deviations from ideal behaviour and the vapour pressure of each component is considerably less than that predicted by Raoult’s law, for these type of solutions.
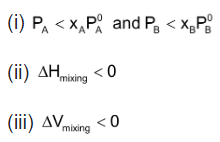
Negative deviation
The solution of HNO3 in water shows negative deviation. Attractive forces between A = A or B − B < A − B
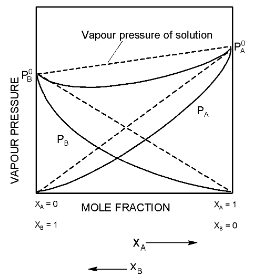
Negative deviations occurs when the interaction between unlike molecules is stronger than the interaction between like molecules. The solutions of this type are characterized by negative enthalpy of mixing and negative volume of mixing.
For example - acetone - chloroform, water - sulphuric acid and water - nitric acid.
