
Depression Of Freezing Point By A Non Volatile Solute
Liquid Solution of Class 12
Depression Of Freezing Point By A Non Volatile Solute
The temperature at which solid and liquid states of a substance have the same vapour pressure is called freezing point. If a non volatile solute is mixed with a pure solvent, the freezing point of pure solvent always greater than the impure one.
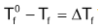 where Tf0 is the freezing point of the pure solvent and Tf is the freezing point of a non – volatile solute containing solvent and ΔTf is the depression in freezing point.
where Tf0 is the freezing point of the pure solvent and Tf is the freezing point of a non – volatile solute containing solvent and ΔTf is the depression in freezing point.
ΔTf ∝ molality
or ΔTf = Kf

Kf = molal freezing point depression constant of the solvent (cryoscopic constant)
m = molality of the solution
Molal freezing point depression constant of the solvent or cryoscopic constant is defined as the depression in freezing point which may be theoretically produced by dissolving 1 mole of any Non volatile non electrolyte solute in 1000 gm of solvent.
or, 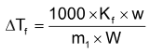
Where m1 = molecular weight of solute
w = weight of solute
W = weight of solvent
