
Group Displacement Law
Chemical Kinetics of Class 12
According to this law, "When an α - particle is emitted the daughter element has atomic number 2 units less than that of the parent element. It is consequently displaced two places to the left in the periodic table. When a β-particle is emitted, the daughter element has an atomic number 1 unit higher than that of the parent element. It is consequently displaced one place to the right in the periodic table.
Counting of number of α and β particles in a radioactive transformation
Parent element Daughter element

Number of α - particles = 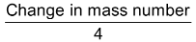 =
= 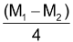
Number of β-particles: Let 'x' β-particles and 'y' α - particles be emitted
Atomic number of parent element – 2y + x = Atomic number of daughter element
Z1 – 2y + x = Z2
∴x = (Z2 – Z1 + 2y)
Further Reading :
