
Molecularity
Chemical Kinetics of Class 12
A chemical reaction that takes place in one and only one step i.e., all that occurs in a single step is called elementary reaction while a chemical reaction occurring in the sequence of two or more steps is called complicated reaction. The sequence of steps through which a complicated reaction takes place is called the reaction mechanism. Each step in a mechanism is an elementary step reaction.
The molecularity of an elementary reaction is defined as the minimum number of molecules, atoms, or ions of the reactants required for the reaction to occur and is equal to the sum of the stoichiometric coefficient of the reactants in the chemical equation of the reaction. Thus, the molecularity of some elementary reactions is as mentioned below:
Elementary reactions Molecularity
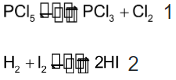
Reactions with molecularity equal to one, two, three, etc; are called unimolecular, biomolecular, trimolecular, etc. respectively.
A complicated reaction has no molecularity of its own but the molecularity of each of the steps (elementary reactions) involved in the mechanism.
For example; consider the reaction;  which is complicated reaction and takes place in the sequence of following three steps:
which is complicated reaction and takes place in the sequence of following three steps:
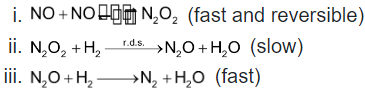
The molecularity of each step in the mechanism is two so we say that the reaction takes in a sequence of three steps each of which is bimolecular. There is another way also. According to which molecularity of a complicated reaction is taken to be equal to the molecularity of the slowest step i.e. rate-determining step (r.d.s) in the mechanism.
For example, the reaction 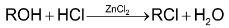
is said to be unimolecular nucleophilic substitution (SN1). Since the reaction occurs in the sequence of the following three steps and the slowest step i.e. r.d.s. is unimolecular.
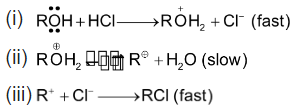
Reactions of higher molecularity (molecularity > 3) are rare. This is because a reaction takes place by a collision between reactant molecules and a number of reactant molecules i.e. molecularity increases the chance of their coming together and colliding simultaneously decreases.
