
Law Of Mass Action
Chemical Kinetics of Class 12
“At a given temperature, the rate of a reaction at a particular instant is proportional to the product of the active masses of the reactants at that instant raised to powers which are numerically equal to the numbers of their respective molecules in the stoichiometric equation describing the reaction”.
Active mass = molar concentration of the substance
= number of gm moles of the substance/Volume in litre
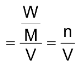
where W = mass of substance, M = molecular mass in grams.
V = volume in litres
Consider a simple reaction 
If CA is the molar concentration or active mass of A at a particular instant, then
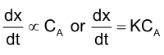
Where K is a proportionality constant or rate constant.
If CA = 1 then
Rate 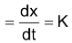
Let us consider a general reaction
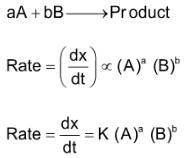
If [A] = [B] = 1 mole / lit, then
Rate = K
Rate of reaction at unit concentration of reactant is called rate constant.
The value of rate constant depends on :
- Nature of reactant
- Temperature
- Catalyst
