
Order Of Reaction
Chemical Kinetics of Class 12
The mathematical expression showing the dependence of rate on the concentration of reactant is known as rate law or rate – expression of the reaction and sum of the indices (powers) of the concentration terms appearing in the rate law as observed experimentally is called order of reaction. To understand what is order of reaction, consider the reaction :  Kinetic experiment carried out at 1100º K upon this reaction has shown following rate data.
Kinetic experiment carried out at 1100º K upon this reaction has shown following rate data.
| Experiment Number | [NO](mole dm-3) |
[H2](mole dm-3) |
Rate (mole dm-3 s-1) |
| 1 | 5 x 10-3 | 2.5 x 10-3 | 3 x 10-5 |
| 2 | 1 x 10-2 | 2.5 x 10-3 | 1.2 x 10-4 |
| 3 | 1 x 10-2 | 5 x 10-3 | 2.4 x 10-4 |
From the experiment number 1 and 2, it is evident that rate increases 4 fold when conc. of NO is doubled keeping the conc. of H2 constant i.e. 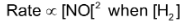 is constant again from experiment number 2 and 3, it is evident that when concentration of H2 is doubled keeping the conc. of NO constant, the rate is just doubled i.e.
is constant again from experiment number 2 and 3, it is evident that when concentration of H2 is doubled keeping the conc. of NO constant, the rate is just doubled i.e. 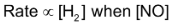 is constant
is constant 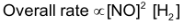
Order of reaction with respect to NO is 2 and with respect to H2 is 1 The overall order of reaction is 2 + 1 = 3. This order of reaction suggest that the reaction is complicated and it does not occur in single step. In order to explain this reaction following mechanism has been proposed.
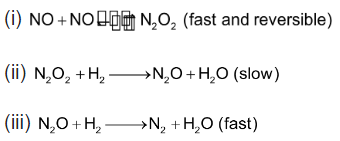
Rate of overall reaction = Rate of step II = K[N2O2][H2] where K = Rate constant of step II N2O2 being intermediate for the overall reaction, its concentration has to be evaluated in terms of the concentration of reactant and this can be done by applying law of mass action upon the equilibrium of step I.
Thus, 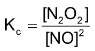 or [N2O2] = Kc[NO]2
or [N2O2] = Kc[NO]2
where Kc=equilibrium constant of step I, putting this value of concentration of N2O2 in the above rate expression, we get 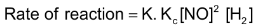 or Rate of reaction
or Rate of reaction  Rate of reaction
Rate of reaction 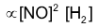 Where K' = K.KC is another constant, rate constant of overall reaction. In general, if rate law of a reaction represented by the equation.
Where K' = K.KC is another constant, rate constant of overall reaction. In general, if rate law of a reaction represented by the equation.  is experimentally found to be as follows :
is experimentally found to be as follows : 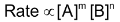 Then order w.r.t. A = m, order w.r.t. B = n Overall order = m + n It may be noted that ‘m’ may or may not be equal to a and similarly ‘n’ may or may not be equal to b, m and n are experimental values, which really depends upon reaction mechanism and experimental condition, may not be predicted by just seeing the chemical equation of the reaction. An example of this is as follows:
Then order w.r.t. A = m, order w.r.t. B = n Overall order = m + n It may be noted that ‘m’ may or may not be equal to a and similarly ‘n’ may or may not be equal to b, m and n are experimental values, which really depends upon reaction mechanism and experimental condition, may not be predicted by just seeing the chemical equation of the reaction. An example of this is as follows:
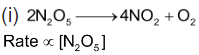 Order of reaction is 1.
Order of reaction is 1.
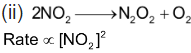 Order of reaction is 2.
Order of reaction is 2.
Further Reading :
Pseudo first order reaction
Reaction whose actual order is different from that expected using rate law expression are called pseudo – order reactions,
eg. 
Expected rate law :
Rate = K[RCI][H2O] , Expected order = 1 + 1 = 2
Actual rate law :
Rate = K'[RCI], Actual order = 1 Water is taken in excess; therefore, its concentration may be taken constant. This reaction is therefore, pseudo first order. Similarly, the acid catalysed hydrolysis of ester, viz,
 follows first order kinetics.
follows first order kinetics.
Rate = K[RCOOR']
It is also a pseudo first order reaction.
Difference between order and molecularity
- Order is an experimental property while molecularity is the theoretical property.
- Order concerns with kinetics (rate law) while molecularity concerns with mechanism.
- Order may be any number, fraction, integral or even zero whereas molecularity is always an integer expecting zero.
