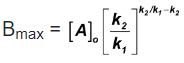Some Complex First Order Reactions
Chemical Kinetics of Class 12
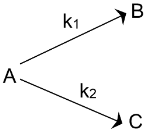
Parallel Reactions:
|
|
In such reactions (mostly organic) a single reactant gives two products B and C with different rate constants. If we assume that both of them are first order, we get. |
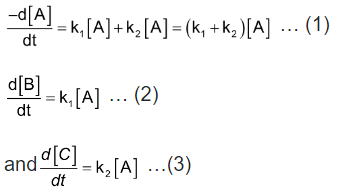
Let us assume that in a time interval, dt, x moles / lit of B was produced and y moles / lit of C was produced.

∴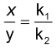 . This means that irrespective of how much time is elapsed, the ratio of concentration of B to that of C from the start (assuming no B and C in the beginning) is a constant equal to k1/k2.
. This means that irrespective of how much time is elapsed, the ratio of concentration of B to that of C from the start (assuming no B and C in the beginning) is a constant equal to k1/k2.
Sequential Reactions:
A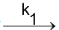 B
B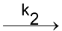 C. In this A decomposes to B which in turn decomposes to C.
C. In this A decomposes to B which in turn decomposes to C.

Integrating equation (1), we get
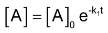
Now we shall integrate equation (2) and find the concentration of B related
to time t.
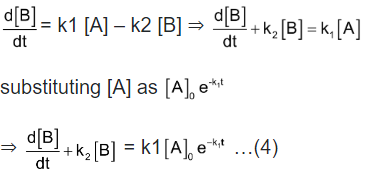
Integration of the above equation is not possible as we are not able to separate the two variables, [B] and t. Therefore we multiply equation (4) by an integrating factor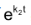 , on both the sides of the equation.
, on both the sides of the equation.
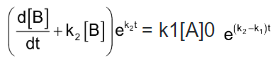
We can see that the left hand side of the equation is a differential of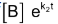 .
.
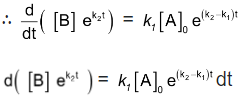
Integrating with in the limits 0 to t.
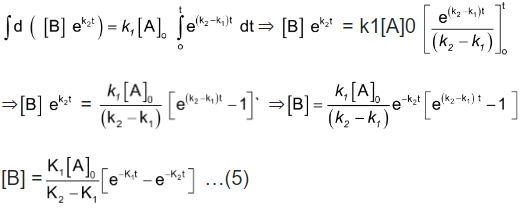
Now in order to find [C], substitute equation (5) in equation (3), we get
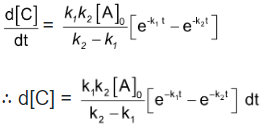
On integrating
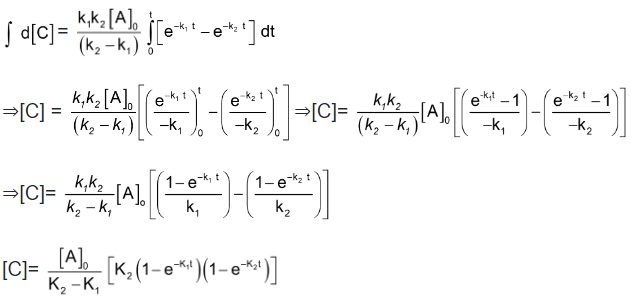
Bmax and tmax: We can also attempt to find the time when [B] becomes maximum. For this we differentiate equation (5) and find 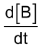 & equate it to zero.
& equate it to zero.
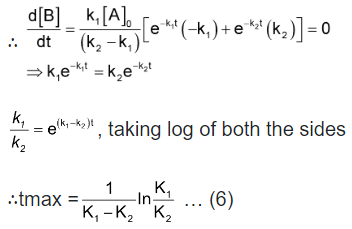
Substituting equation (6) in equation (5)