Enthalpy Of A System
Thermodynamics of Class 11
The quantity U + PV is known as the enthalpy of the system and is denoted by H. It represents the total energy stored in the system. Thus
H = U + PV
It may be noted that like internal energy, enthalpy is also an extensive property as well as a state function. The absolute value of enthalpy can not be determined, however the change in enthalpy can be experimentally determined.
ΔH = ΔU + Δ(PV)
Various kinds of processes:
(i) Isothermal reversible expansion of an Ideal gas: Since internal energy of an Ideal gas is a function of temperature and it remains constant throughout the process hence
ΔE = 0 and ΔH = ΔE + ΔPV
∵ΔE = 0
and P1V1 = P2V2 at constant temperature for a given amount of the gas
∴ ΔH= 0
Calculation of q and w:
∵ ΔE = q + w
For an Isothermal process, w = -q
This shows that in an Isothermal expansion, the work done by the gas is equal to amount of heat absorbed.
and w = - n RT ln(V2/V1) = - n RT ln(P1/P2).
(ii) Adiabatic Reversible Expansion of an Ideal gas:
∵ q = 0
∴ ΔE= -w.
Total change in the internal energy is equal to external work done by the system.
∴Work done by the system = ΔE= CvΔT.
and Cp-Cv = R
On dividing all the terms by Cv.
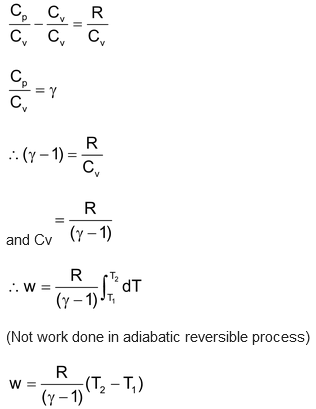
and ΔH = ΔE + PΔV.
Thus if T2>T1, w = +ve i.e. work is done on the system.
Thus if T2<T1, w = -ve i.e. work is done by the system.
Limitations of the first law. Need for the second law
A major limitation of the first law of thermodynamics is that its merely indicates that in any process there is an exact equivalence between the various forms of energies involved, but it provides no information concerning the spontaneity or feasibility of the process. For example, the first law does not indicate whether heat can flow from a cold end to a hot end or not.
The answers to the above questions are provided by the second law of thermodynamics.
Spontaneous and non – spontaneous process
If in the expansion of a gas the opposing pressure is infinitesimally smaller than the pressure of the gas, the expansion takes place infinitesimally slowly i.e. reversible. If however, the opposing pressure is much smaller than the pressure of the gas the expansion takes place rapidly i.e. irreversibly. Natural processes are spontaneous and irreversible.
- Introduction
- Some Basic Terms
- Isochoric Process
- Internal Energy, U
- Mathematical Expression Of First Law
- Enthalpy Of A System
- Second Law Of Thermodynamics
- Gibbs Free Energy
- Relationship Between Free Energy And Equilibrium Constant
- Third Law Of Thermodynamics
- Thermochemistry
- Hess's Law
- Lattice Energy Of An Ionic Crystal (Born–Haber Cycle)
- Bomb Calorimeter
- Heat Capacity And Specific Heat
- Variation Of Heat Of Reaction With Temperature
- Exercise 1
- Exercise 2









