Lattice Energy Of An Ionic Crystal (Born–Haber Cycle)
Thermodynamics of Class 11
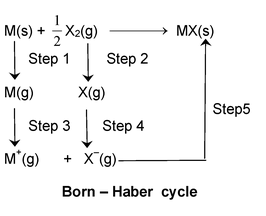
The change in enthalpy that occurs when 1 mole of a solid crystalline substance is formed from its gaseous ions, is known as Lattice energy.
Step 1: Conversion of metal to gaseous atoms
M(s) → M(g) , ΔH1 = sublimation
Step 2: Dissociation of X2 molecules to X atoms
X2(g) → 2X (g), ΔH2 = Dissociation energy
Step 3: Conversion of gaseous metal atom to metal ions by losing electron
M(g) → M+ (g) + e–, ΔH3 = (Ionization energy)
Step 4: X(g) atoms gain an electron to form X– ions
X(g) + e– → X–(g), ΔH4 = Electron affinity
Step 5: M+ (g) and X– (g) get together and form the crystal lattice
M+ (g) + X– (g) → MX(s) ΔH5 = lattice energy
Applying Hess’s law we get
ΔH1 + 1/2 ΔH2 + ΔH3 + ΔH4 + ΔH5 = ΔHf (MX)
On putting the various known values, we can calculate the lattice energy.
- Introduction
- Some Basic Terms
- Isochoric Process
- Internal Energy, U
- Mathematical Expression Of First Law
- Enthalpy Of A System
- Second Law Of Thermodynamics
- Gibbs Free Energy
- Relationship Between Free Energy And Equilibrium Constant
- Third Law Of Thermodynamics
- Thermochemistry
- Hess's Law
- Lattice Energy Of An Ionic Crystal (Born–Haber Cycle)
- Bomb Calorimeter
- Heat Capacity And Specific Heat
- Variation Of Heat Of Reaction With Temperature
- Exercise 1
- Exercise 2









