Explain the mechanism of Bromination of Phenol
Bromination phenol
Phenol on treatment with chlorine or bromine water gives an immediate precipitate of 2,4,6−trihalogen derivative, when treated with bromine water it gives bromination of phenol. Phenol in aqueous medium is partially ionised and the phenoxide ion thus obtained is much more reactive than phenol itself towards electrophilic attack. Moreover, halogen reacts with water forming halogen acid and hypohalous acid. The proton attacks at the OH group of hypohalous acid to give H2O⊕−X, which acts as a stronger electrophile. Therefore, halogenation takes place at all the ortho and para positions. Check out Chemistry Formulas and NCERT Solutions for class 11 Chemistry prepared by Physics Wallah.

Br2 + H2O 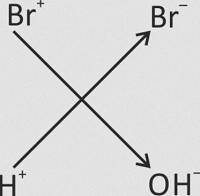 H−Br + HO−Br
H−Br + HO−Br 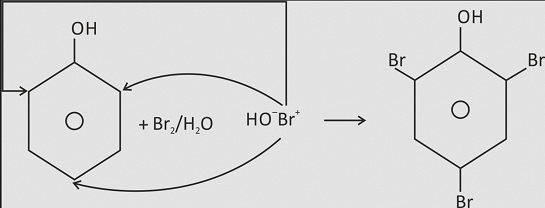 HO−Br
HO−Br
If the halogenation is carried out in non-aqueous medium like CS2 or CCl4, only mono−substitution takes place. This is because the ionisation of phenol does not occur in non-aqueous medium. The benzene ring of phenol is less activated than that of phenoxide ion as well as the bromine in Br2 molecule is not as electrophilic as in 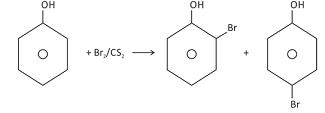
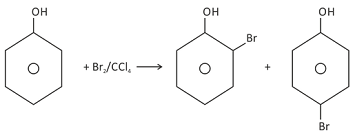
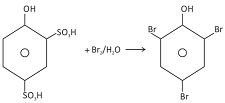
o−Bromophenol is also prepared by protecting one ortho and the para positions by sulphonation.
Mechanism of Bromination
So strong is the activation of benzene ring in aqueous medium that derivatives of phenol containing −COOH group or −SO3H group either at the ortho or at the para position are displaced by Br in bromination reaction. This is an example of brominative decarboxylation. For example, salicylic acid when treated with bromine water gives 2,4,6−trisubstituted phenol.
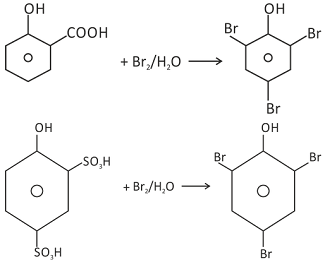
In case when SO3H group is present at ortho and para positions, desulphonation takes place liberating SO3 gas.
Trisubstitution of benzene is also observed when aniline is treated with bromine because the reactivity of aniline is same as that of phenoxide ion towards electrophilic attack.
Recent Concepts
- How to calculate equivalent weight ?
- What is equivalent weight of oxalic acid
- Atomic weight of elements
- What is Limiting Reagents ?
- Molarity
- Define back bonding in BF3
- Heisenberg's Uncertainty Principle
- What are Inert pair effects
- Quantum Numbers
- What is the boron trifluoride formula?
- What is the Structure of orthoboric acid ?
- What is the Structure of Borax ?
- What is the structure of diboranes ?
- LiAlH4 Reaction and Mechanism
- Grignard reagent and its application in organic reactions
- How Haloform reaction proceed ?
- What is Oxirane ?
- Acidity of Phenols
- Explain the mechanism of Bromination of Phenol
- what is a chemical reaction?









