what is a chemical reaction?
What is A Chemical Reaction ?
Let us consider the following process occurring in our daily life:
-
Melting of ice,
-
Evaporation of water,
-
Dissolution of sugar in water,
-
Rusting of iron articles such as tava or pan or nails etc. especially in the rainy season,
-
Souring of milk in summer, and
-
Burning of coke in air.
The first three process, (i) to (iii), do not lead to the formation of any new chemical substances Check out Chemistry Formulas and NCERT Solutions for class 11 Chemistry prepared by Physics Wallah.
For example; ice, liquid water and water vapour are chemically all same, i.e., water (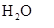 ). Solution of sugar in water still contain the same chemical substance, i.e., sugar and water
). Solution of sugar in water still contain the same chemical substance, i.e., sugar and water
Such processes in which no new substances are formed are called physical changes.
In process (iv) to (vi), the original substances lose their nature and identity and form new chemical substance with different properties are called chemical changes . The process involving a chemical change is called a chemical reaction.
A few more examples of processes occurring in everyday life and involving chemical changes are given:
-
Turning brown of a freshly cut apple in the air,
-
Cooking of food
-
Digestion of food in our body,
-
Respiration,
-
Fermentation of grapes,
-
Burning of candle wax, and
-
Burning of fuels like kerosene oil, petrol, LPG etc.
What are reactant and products?
The chemical substances taken originally are called reactants and the new chemical substances formed are called products. For example, in the burning of coke in air or oxygen, carbon and oxygen are the reactants while carbon dioxide formed is the product.
A chemical reaction involves breaking of chemical bonds present in the reactant molecules forming new chemical bonds to give the products. Thus a chemical reaction simply involves rearrangement of atoms. The atoms of an element themselves do not undergo any changes to form atoms of a new element.
Examples of some chemical reactions
Here, we discuss examples of some chemical reactions which can be easily carried out in the laboratory .
Examples 1. Burning of magnesium ribbon in the air
Magnesium metal is generally available in the laboratory in the form of a ribbon or a wire. It has a shining surface. However, due to reaction with moist air, it is coated with a white layer of magnesium oxide. The experiment is carried out as follows:
Clean a piece of magnesium ribbon by rubbing with a sand paper to remove the layer of magnesium oxide. Hold it in a pair of tongs , now , burn it in the air with a spirit lamp or a burner , keeping a china dish below as shown in the fig. 1.1. we observe that magnesium burns with a dazzling light and a white powder is obtained in the china dish . The white powder is found to be that of magnesium oxide MgO. Thus, magnesium has combined with oxygen of the air to form a new chemical substance, magnesium oxide. Hence, we say that a chemical reaction has taken place in which magnesium and oxygen are the reactants while magnesium oxide is the product.
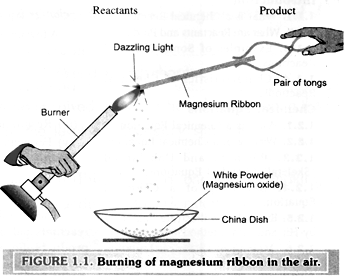
Caution: The dazzling light produced on the burning magnesium ribbon can be harmful to the eyes. Hence, while performing this experiment, keep your eyes as far away as possible from the burning magnesium ribbon, better wear some eye protection during the experiment.
Example 2: Reaction Between Lead Nitrate And Potassium Iodide.
If we prepare lead nitrate solution in one test tube and potassium iodide solution in another test tube and then mix the two solutions, we observe that a yellow solid (called precipitate) appears. This yellow solid is a new chemical compound, namely, lead iodide. Another substance formed is potassium nitrate which we cannot see as it remains in the solution. Here again , a chemical reaction has taken place in which lead nitrate and potassium iodide are the reactants while lead iodide and potassium nitrate are the products.
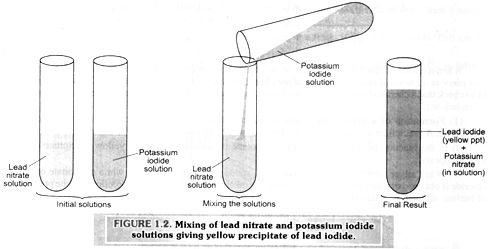
Examples 3: Reaction between zinc and dilute sulphuric acid (hydrochloric acid).
If we take a few pieces of granulated zinc in small conical flask or a test tube and add dilute sulphuric acid (or hydrochloric acid) into it, we observe that from the surface of the zinc pieces, a gas is evolved briskly. Further, if we touch the flask or the test tube, it is found to be hot. To test the gas evolved, the flask is fitted with a tube as shown in fig. 1.3.
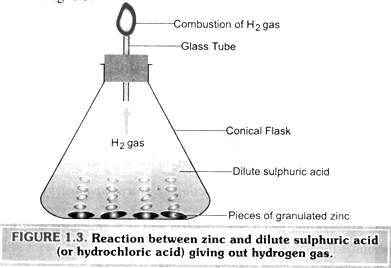
On bringing a lighted candle near the upper end of the tube, the gas is found to burn with a popping sound. Thus, the gas evolved is hydrogen.
Another substance formed is zinc sulphate (or zinc chloride) which we cannot see because it remains in the solution. Here, again a chemical reaction has taken place in which zinc and sulphuric acid (or hydrochloric acid) are the reactants while hydrogen gas and zinc sulphate (or zinc chloride) are products.
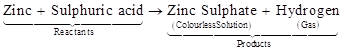
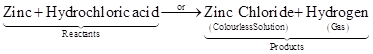
Recent Concepts
- How to calculate equivalent weight ?
- What is equivalent weight of oxalic acid
- Atomic weight of elements
- What is Limiting Reagents ?
- Molarity
- Define back bonding in BF3
- Heisenberg's Uncertainty Principle
- What are Inert pair effects
- Quantum Numbers
- What is the boron trifluoride formula?
- What is the Structure of orthoboric acid ?
- What is the Structure of Borax ?
- What is the structure of diboranes ?
- LiAlH4 Reaction and Mechanism
- Grignard reagent and its application in organic reactions
- How Haloform reaction proceed ?
- What is Oxirane ?
- Acidity of Phenols
- Explain the mechanism of Bromination of Phenol
- what is a chemical reaction?









