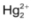Alkaline Earth Metals
S and P Block Elements of Class 11
Alkaline Earth Metals
The group 2 of the periodic table consists of six metallic elements. They are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). The name alkaline earth metals was given to magnesium, calcium, barium & strontium since their oxides were alkaline in nature and these oxide remained unaffected by heat or fire and existed in earth.
Occurrence
Like alkali metals, alkaline earth metals are also highly reactive and hence do not occur in the free state but are likely distributed in nature in the combined state as silicates, carbonates, sulphates and phosphates.
Minerals
Be – Beryl (Be3Al2Si6O18) & Phenacite (Be2SiO4)
Mg – Magnesite MgCO3, Dolomite CaMg(CO3)2, Epsomite MgSO4.&H2O
Ca – Limestone (CaCO3), fluoropatite [3(Ca3(PO4)3.CaF2], Gypsum (CaSO4.2H2O), Anhydrite (CaSO4)
Sr – Celestite (SrSO4), Strontianite (SrCO3)
Br – Barytes (BaSO4)
Electronic Configuration
The general electronic configuration of alkaline earth metals is ns2.
Be – 1s22s2 Mg – 1s22s2sp63s2
Ca – 1s22s22p63s23p64s2 Sr – [Kr]5s2
Ba – [Xe]6s2 Ra – [Rn]7s2
Physical Properties of Group II elements
(i) Atomic and ionic radii
The atomic radii as well as ionic radii of the members of the family are smaller than the corresponding members of alkali metals.
(ii) Ionization energy
The alkaline earth metal owing to their large size of atoms have fairly low values of ionization energies as compared to the p – block elements. However with in the group, the ionization energy decreases as the atomic number increases. It is because of increase in atomic size due to addition of new shells and increase in the magnitude of screening effect of the electrons in inner shells. Because their (IE)1 is larger than that of their alkali metal neighbours, the group IIA metals trend to the some what less reactive than alkali metals. The general reactivity trend is Ba > Sr > Ca > Mg > Be.
(iii) Oxidation state
The alkaline earth metal have two electrons in their valence shell and by losing these electrons, these atoms acquire the stable noble gas configuration. Thus, unlike alkali metals, the alkaline earth metals exhibit +2 oxidation state in their compounds.

Lattice energies decreases as atomic number increases
|
MO |
MCO3 |
MF2 |
|
|
Mg |
-3923 |
-3178 |
-2906 |
|
Ca |
-3517 |
-2986 |
-2610 |
|
Sr |
-3312 |
-2718 |
-2459 |
|
Ba |
-3120 |
-2614 |
-2367 |
(v) Nature of metallic bonding in alkaline earth metals
The alkali metal two electrons are involved in the metallic bonding. Moreover, sizes of alkaline earth metal ions are smaller than those of alkali metal ions. Consequently, stronger metallic bonds are formed which result in the close packing of the atoms. Due to the presence of stronger metallic bonds, alkaline earth metals have
(a) Higher melting points (b) Higher boiling points
(c) higher densities (d) Harder than the corresponding alkali metals.
(vi) Density
The alkaline earth metals are denser and harder than the corresponding alkali metals.
The atoms of alkaline earth metals have smaller size and are hence held by stronger metallic bonds, as compared to alkali metals. Therefore, they are more closely packed in their crystal lattice which accounts for high density and increased hardness of these elements.
(vii) Characteristic flame colouration
Expect Be & Mg (due to high ionization energy), the alkaline earth metals impart characteristic colour when introduced into flame of a burner. This property is due to the ease of excitation of their valence electrons. When elements or their compounds are introduced to flame, the electron absorbs energy from the flame and gets excited to higher energy levels. When these electrons return to their ground state, they emit absorbed energy in form of visible light having characteristic wavelengths. Depending upon the wavelength of light emitted, different colours are impart to the flame. Salts (generally chlorides) impart characteristic colours to the Bunsen flame.
|
Ion |
Colour |
|
Ca2+ |
Brick-red |
|
Sr2+ |
Crimson |
|
Ba2+ |
Apple green |
|
Ra2+ |
Carmine – red |
Electropositive or Metallic Character
The alkaline earth metals are highly electropositive and hence metallic and their electropositive or metallic character increases down the group. However they are less electropositive or metallic than the alkali metals. It is due to smaller size and higher ionization energies as compared to alkali metals, hence have less tendency to loose electron than those of alkali metals (group I)
Like the alkali metals they also form predominantly ionic compounds but tendency of covalency is greater, particularly with Be and Mg because of their smaller atomic and ionic radii. Be forms compounds which are essentially covalent.
Melting and boiling points
The alkaline earth metals have higher melting and boiling points as compared to those of alkali metals which is attributed to their small size and more close packed crystal lattice as compared to alkali metals and presence of two valence electrons.
Heat of Hydration
-
The heats of hydration of M2+ decreases with an increase in their ionic size and their values are greater than that of alkali metal ions.
-
Alkaline earth metal ions, because of their larger charge to size ratio, exert a much stronger electrostatic attraction on the oxygen of water molecule surrounding them.
-
Since the alkaline earth metals (except Be) tend to lose their valence electrons readily, they act as strong reducing agents as indicated by E0red values. The particularly less negative value for Be arises from the large hydration energy associated with the small size of Be2+ and the relatively large value of heat of sublimation.
Solubility
-
Basic nature of oxides increases down the group but solubilities of sulphates and carbonates decrease as ionic size increases.
-
The solubility of most salts decreases with increased atomic weight, though usual trend is reversed with fluorides and hydroxides in this group.
Physical Properties of groups 2 elements (alkaline earth metals)
|
Property |
Elements |
|||||
|
Be |
Mg |
Ca |
Sr |
Ba |
Ra |
|
|
Atomic number |
4 |
12 |
20 |
38 |
56 |
88 |
|
Atomic mass |
9.01 |
24.31 |
40.08 |
87.62 |
137.33 |
226.03 |
|
Metallic radius/pm |
112 |
160 |
197 |
215 |
222 |
- |
|
Ionic radius/pm |
51 |
72 |
100 |
118 |
135 |
148 |
|
Ionization enthalpy I (kJ mol−1) II |
899 1757 |
737 1450 |
590 1146 |
549 1064 |
503 965 |
509 979 |
|
Enthalpy of hydration of M2+ ions (kJ mol−1) |
−2494 |
−1921 |
−1577 |
−1443 |
−1305 |
- |
|
Electronegativity (Pauling Scale) |
1.57 |
1.31 |
1.00 |
0.95 |
0.89 |
0.9 |
|
Density/g mol− at 298 K |
1.85 |
1.74 |
1.55 |
2.63 |
3.62 |
5.5 |
|
Melting Point/K |
1562 |
924 |
1124 |
1062 |
1002 |
973 |
|
Boiling point /K |
2745 |
1363 |
1767 |
1655 |
2078 |
(1973) (uncertain) |
|
E°(V) at 298 K for M2+(aq) + 2e− → M(s) |
−1.97 |
−2.37 |
−2.87 |
−2.89 |
−2.90 |
−2.92 |
|
Occurrence in Lithosphere |
2* |
2.76** |
4.6** |
384* |
390* |
10−10** |
* ppm (parts per million) ** Percentage by weight
Reactivity and Electrode potential
All the alkaline earth metals are highly reactive elements since they have a strong tendency to lose the two valences s-electrons to form the corresponding dipositive ions having inert gas configuration. The high reactivity arises due to their low ionization energies and high negative values of their standard electrode potentials. Further, the chemical reactivity of alkaline earth metals increase on moving down the group because the I.E. decreases and electrode potentials become more and more negative with increasing atomic number from Be to Ra. Thus, beryllium is the least reactive while Ba (or Ra) is the most reactive element. Further since the ionization energies of alkaline earth metals are higher and their electrode potential is less negative than the corresponding alkali metals. They are less reactive than corresponding alkali metals.
Reducing Character
The alkaline earth metals are weaker reducing agents than the alkali metals. Like alkali metals, their reducing character also increases down the group. This is due to the reason that the alkaline earth metals have greater tendency to lose electrons so, they act as reducing agent but since their I.E. are higher and their electrode potentials are less negative than the corresponding alkali metals, therefore alkaline earth metals are weaker reducing agents than alkali metals. The sulphates are stable to heat whereas the carbonates decompose to give MO and CO2, the temperature of decomposition increasing from Mg to Ba. BeCO3 is kept in the atmosphere of CO2 to prevent its decomposition.
BeCO3 MgCO3 CaCO3 SrCO3 BaCO3
<100°C 540°C 900°C 1290°C 1360°C
Occurrence and uses of alkaline earth metals
|
Elements |
Abundance |
Main Minerals |
Uses |
|
Beryllium |
2.8 × 10−3% |
First detected in 1798 in the gemstone beryl and emerald (Be2Al2Si6O18) |
Used in corrosion resistant alloys. |
|
Magnesium |
2.33%, 7th most abundant element in earth’s crust |
Pure Mg first prepared in 180, named after the magnesia district in Thessaly Greece where large deposits of the mineral are found |
When alloyed with Al, Mg is widely used as structural materials because of its high strength, low density and ease in machining. |
|
Calcium |
4.15%, 5th most abundant element in earth’s crust. |
CaCO3.2H2O obtained in pure form in 1808, calcium is derived from latin word calx, meaning “lime” |
As an alloying agent for hardness in aluminium compounds. Calcium is the primary constituent of teeth and bones. |
|
Strontium |
0.038% |
Discovered in 1787 and named after the small town of strontion (Scotland) |
SrCO3 is used for the manufactured of glass for colour TV picture tubes. |
|
Barium |
0.042% |
Found in minerals witherite (BaCO3) and barite (BaSO4) after which it is named. |
BaSO4 is used in medicine as a contrast medium for stomach and intestine X – rays |
|
Radium |
Traces |
Isolated as chloride in 1898 from the mineral pitchblende |
Used in cancer radiotheraphy |
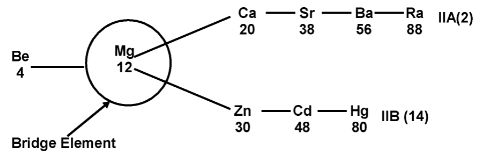
Group IIA (Alkaline earth metals) and groups IIB (Zn, Cd, Hg) Mg acts as a bridge element between IIA and IIB.
|
|
|||||
|
Sr.No. |
Properties |
IIA(Be, Mg, Ca, Sr, Ba, Ra) |
IIB (Zn, Cd, Hg) |
||
|
1 |
Electronic configuration |
[Inert gas] ns2 |
[Inert gas] (n – 1)d10ns2 |
||
|
2. |
Block |
S – block |
d – block |
||
|
3. |
Oxidation state |
+2 |
+2, mercury also forms dimeric |
||
|
4. |
Nature of oxide |
BeO is amphoteric, other oxides are basic. |
ZnO is amphoteric, CdO and MgO are basic |
||
|
5. |
Nature of Halides |
Electron – deficient BeX2, others (MX2) are ionic: MgCl2 < CaCl2 < SrCl2 < BaCl2 |
ZnCl2, CdCl2 are ionic but less than IIA, HgCl2 is covalent. |
||
|
6. |
Nature of sulphates |
Less soluble in water and solubility decreases down the group BeSO4 > MgSO4 > CaSO4 > SrSO4 > BaSO4 |
More soluble than IIA |
||
|
7. |
Nature of hydroxides |
Solubility of hydroxides increases as we move down the group. |
Solubility of hydroxides decrease as we move down the group. |
||
|
8. |
Nature of sulphides |
Soluble |
ZnS, CdS, HgS insoluble and precipitate in salt analysis. |
||
|
9. |
Reactivity |
Increases as we move down the group Be < Mg < Ca < Sr < Ba |
Decreases as we move down the group Zn > Cd > Hg |
||
Difference between alkaline earth metals and alkali metals
Both alkaline earth metals and alkali metals are s – block element as the last electron enters the ns – orbital. They resemble with each other in some respects but still there are certain dissimilarities in their properties on account of different number of electrons in the valency shell, smaller atomic radii, high ionization potential, higher electro negativity etc.
|
Properties |
Alkaline earth metals |
Alkali metals |
|
|
1. |
Electronic configuration |
Two electrons are present in the valency shall. The configuration is ns2 (bivalent) |
One electron is present in the valency shell. The configuration is ns1 (monovalent) more electropositive |
|
2. |
Valency |
Bivalent |
Monovalent |
|
3. |
Electropositive nature |
Less electropositive |
More electropositive |
|
4. |
Hydroxides |
Weak bases, less soluble and decompose on heating. |
Strong bases, highly soluble and stable towards heat. |
|
5. |
Bicarbonates |
These are not known in free state. Exist only in solution. |
These are known in solid state. |
|
6. |
Carbonates |
Insoluble in water. Decompose on heating. |
Soluble in water. Do not decompose on heating (LiCO3 is an exception) |
|
7. |
Action of nitrogen |
Directly combine with nitrogen and form nitrides |
Do not directly combine with nitrogen except lithium |
|
8. |
Action of carbon |
Directly combine with carbon and form carbides |
Do not directly combine with carbon |
|
9. |
Nitrates |
Decompose on heating evolving a mixture of NO2 and oxygen |
Decompose on heating evolving only oxygen |
|
10. |
Solubility of salts |
Sulphates, phosphates fluorides, chromates, oxalates etc are insoluble in water |
Sulphates, phosphates, fluorides, chromates, oxides etc are soluble in water. |
|
11. |
Physical properties |
Comparatively harder. High melting points. Diamagnetic. |
Soft, low melting points paramagnetic. |
|
12. |
Hydration of compounds |
The compounds are extensively hydrated. MgCl2.6H2O, CaCl2.6H2O, BaCl2.2H2O are hydrated chlorides. |
The compounds are less hydrated. NaCl, KCl, RbCl form non – hydrated chlorides |
|
13. |
Reducing power |
Weaker as ionization potential values are high and oxidation potential values are low. |
Stronger as ionization potential values are low and oxidation potential values are high. |
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8