Sodium
S and P Block Elements of Class 11
Sodium is the 7th most abundant element by weight found in earth’s crust.
Minerals of sodium
(i) Rock salt – NaCl
(ii) Chile salt petre – NaNO3
(iii) Glauber’s salt – Na2SO4.10H2O
(iv) Borax or sodium borate – Na2B4O7.10H2O
(v) Albite or soda feldspar – Na2O.Al2O3.6SiO2 or NaAlSi3O8
Extraction of sodium
Sodium is extracted by Down’s process through electrolysis of fused sodium chloride.
In this method, sodium is obtained by the electrolysis of mixture of sodium chloride (40%) and calcium chloride (60%) in fused state. The function of calcium chloride is to lower the operating temperature from 1080 K (m.pt. of NaCl) to about 850 K. The main reason for lowering the temperature are:
(i) The melting point of sodium chloride is very high it is very difficult to maintain it in the molten state during electrolysis.
(ii) Sodium is considerably volatile at the temperature needed for the electrolysis and therefore, a part of the metal produced is vapourised.
(iii) Molten sodium gets dispersed in molten chloride to form a metallic fog (colloidal solution) at high temperature.
(iv) Both sodium and chlorine, the two products of the electrolysis, have a corrosive action on the material of the vessel employed for the electrolysis at such a high temperature.
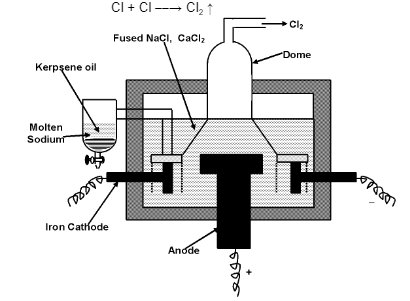
The cell consists of a steel tank lined with fire bricks. A circular graphite anode is placed in the centre of the cell which is surrounded by a cylindrical iron cathode. The anode and cathode are separated by a steel gauge cylinder through which molten sodium chloride can pass but molten sodium cannot. The purpose of using steel gauge is to keep sodium separate from chlorine which would otherwise react with chlorine. The anode is covered by a dome – shaped steel hood which provides the out – let for the escape of chlorine gas. The molten metal liberated at the cathode moves up and flow into the receiver containing kerosene oil. The following reactions take place:
NaCl → Na+ + Cl− (Ionization)
At cathode: Na+ + e− → Na
At node: Cl− → Cl + e−
Cl + Cl → Cl2 ↑
Properties of Sodium
Physical Properties
1. Sodium is soft, silvery white metal.
2. It is lighter than water, its density is 0.97 g/cm3.
3. It imparts golden yellow flame when introduced into Bunsen flame.
Chemical Properties
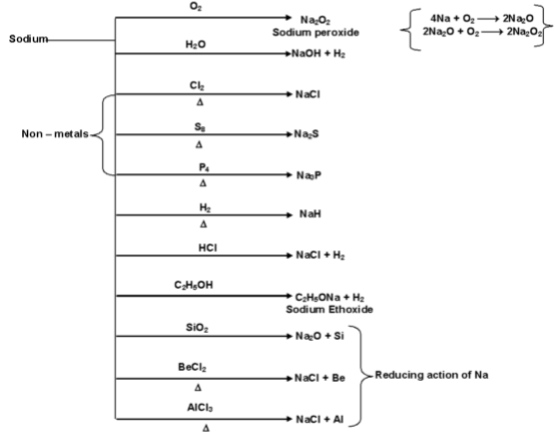
Sodium is more reactive than lithium.
(i) Action of air and moisture
It is tarnished rapidly on exposure to moist air.
First a film of sodium monoxide, Na2O is formed which changes readily into sodium hydroxide by action of moisture and finally into sodium carbonate by the action of CO2 present in air.
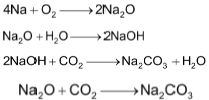
It is therefore kept under kerosene.
Action of ammonia
Sodium dissolves in liquid ammonia to form blue solution which is a good conductor of electricity.

However, when ammonia is passed through molten sodium, it yield sodamide evolving H2 gas.

Uses of sodium
(i) Sodium is used as a reducing agent in the extraction of boron and silicon.
(ii) Liquid sodium or its alloy with potassium is used as coolent in nuclear reactors.
(iii) Sodium as well as sodium amalgam are used as reducing agents in organic synthesis.
(iv) It is used in Lassaigne’s test for the detection of N, S and halogen in organic compounds.
(v) Sodium is used in sodium vapour lamps.
(vi) It is largely used for production of artificial rubber, dyes, drugs etc.
(vii) Sodium-lead alloy is used for preparation of tetraethyl lead. Pb(C2H5)4 which is used as an anti-knocking agent in petrol.
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8









