Chemical Properties Of Group II elements
S and P Block Elements of Class 11
Reaction with water – (Formation of hydroxides)
The electrode potential of Be (Be2+/Be = −1.97 V) is least negative amongst all the alkaline earth metals. This means that Be is much less electropositive than other alkaline earth metals and hence does not react with water or steam even at red heat.
The electrode potential of Mg (Mg+2/Mg = −2.37 V), although more negative than that of Be yet is still less negative than those of alkali metals and hence it does not react with cold water but reacts with boiling water or steam.

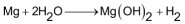
Mg, infact, forms a protective layer of oxide on its surface, therefore, despite its favourable electrode potential it does not react readily with water unless the oxide layer is removed by amalgamating it with mercury. In the formation of oxide film, Mg resembles Al.
Ca, Sr and Ba have more negative electrode potentials similar to those of the corresponding group I alkali metals and hence react with even with cold water, liberating H2 and forming the corresponding metal hydroxides.
Ca + 2H2O→ Ca(OH)2 + H2
Reactivity of alkaline earth metals increases as we move down the group. However, the reaction of alkaline earth metals is less vigorous as compared to alkali metals.
Reaction with air (Nitrogen and Oxygen)
(a) Formation of oxides and nitrides
Be metal is relatively unreactive in the massive form and hence does not react below 873K. However, powdered Be is more reactive and burns brilliantly on ignition to give a mixture of BeO & Be3N2.
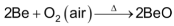
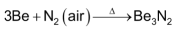
magnesium is more electropositive than Be and hence burns with dazzling brilliance in air to form a mixture of MgO and magnesium nitride.
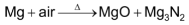
Ca, Sr and Ba being even more electropositive react with air readily to form a mixture of their respective oxides and nitrides.
The reactivity towards oxygen increases as we go down the group. Thus Ca, Ba and Sr are stored in paraffin but Be and Mg are not because they form protective oxide layer on their surface.
(b) Formation of Nitrides
All the alkaline metals burn in dinitrogen to form ionic nitrides of the formula, M3N2. This is in contrast to alkali metals where only Li forms Li3N.
3M + N2 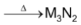
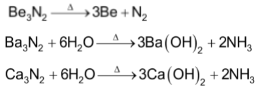
Be3N2 being covalent is volatile while the nitrides of all other elements are crystalline solids.
All these nitrides decompose on heating and react with water liberating NH3.
(c) Formation of Peroxides
Since larger cations stabilize larger anions. Therefore, tendency to form peroxide increases as the size of the metal ion becomes larger. Thus BaO2 is formed by passing air over heated BaO at 773K.
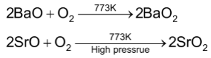
SrO2 is prepared in similar way but under high pressure and temperature. CaO2 is not formed this way but can be prepared as the hydrate by treating Ca(OH)2 with H2O2 and then dehydrating the product.
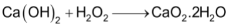
Crude MgO2 has been made using H2O2 but peroxide of beryllium is not known.
All peroxide are white crystalline ionic solids containing the peroxide ion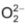 . Treatment of peroxide with acids liberates H2O2.
. Treatment of peroxide with acids liberates H2O2.

Reaction with hydrogen – (Formation of hydrides)
All the alkaline earth metals except Be combine with hydrogen directly on heating to form metal hydrides of formula MH2.
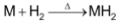
The hydride of beryllium can also be obtained by the reduction of BeCl2 with LiAlH4.
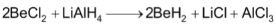
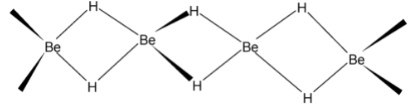
Both BeH2 and MgH2 are covalent compounds having polymeric structures in which H – atoms between beryllium atoms are held together by three centre – two electron (3C − 2e) bonds as shown below:
The hydrides of other elements of this groups i.e. CaH2, SrH2 and BaH2 are ionic and contain the H− ions.
All the hydrides of alkaline earth metals reacts with water liberating H2 gas and thus act as reducing agents.

CaH2 is called Hydrolith and is used for production of H2 by action of water on it.
Reaction with carbon – (Formation of carbides)
When BeO is heated with carbon at 2175 – 2275 K a brick red coloured carbide of the formula Be2C is formed
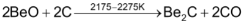 .
.
It is a covalent compound and react water forming methane.
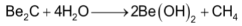
The rest of the alkaline earth metals (Mg, Ca, Sr & Ba) form carbides of the general formula, MC2 either when the metal is heated with carbon in an electric furnace or when their oxides are heated with carbon.
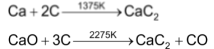
All these carbides react with water producing acetylene gas.
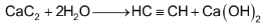
Reaction with Halogens
The alkaline earth metals react with halogens at elevated temperature to form the halides of the types MX2.
Action of Acids
The alkaline earth metals readily react with acids liberating hydrogen.
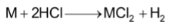
Reaction with Ammonia
Like alkali metal, the alkaline earth metals dissolve in liquid ammonia to give deep blue black solution from which ammoniates can be recovered.
General characteristics of compounds of the Alkaline earth metals
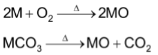
(a) Oxides
The oxides MO are obtained either by heating the metals in oxygen or by thermal decomposition of their carbonates.
Expect BeO all other oxides are extremely stable ionic solids due to their high lattice energies.
These have high melting point, have very low vapour pressure, are very good conducts of heat, are chemically inert and act as electrical insulators. Therefore, these oxides are used for lining furnaces and hence used as refractory materials.
Due to small size of beryllium ion, BeO is covalent but still has high melting point because of its polymeric nature.
(b) Hydroxides
The hydroxides of Ca, Sr & Ba are obtained either by treating the metal with cold water or by reacting the corresponding oxides with water. The reaction of these oxides with H2O is also sometimes called as slaking.
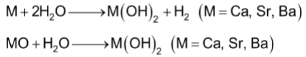
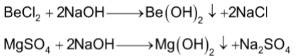
Be(OH)2 and Mg(OH)2 being insoluble are obtained from suitable metal ion solutions by precipitation with OH− ions.
Properties
(i) Basic Character
All the alkaline earth metal hydroxides are bases except Be(OH)2 which is amphoteric. This basic strength increases as we move down the group. This is because of increase in size which results in decrease of ionization energy which weakens the strength of M – O bonds in MOH and thus increase the basic strength. However, these hydroxides are less basic than the corresponding alkali metal hydroxides because of higher ionization energies, smaller ionic sizes and greater lattice energies.
(ii) Solubility in Water
Alkaline earth metals hydroxides are less soluble in water as compared to alkali metals.
The solubility of the alkaline earth metal hydroxides in water increases with increase in atomic number down the group. This is due to the fact that the lattice energy decreases down the group due to increase in size of the alkaline earth metals cation whereas the hydration energy of the cation remains almost unchanged. The resultant of two effects i.e.

becomes more negative as we move from Be(OH)2 to Ba(OH)2 which accounts for increase in solubility.
Halides
The alkaline earth metals combine directly with halogen at appropriate temperature forming halides MX2.
These halides can also be prepared by the action of halogen acids (HX) on metals, metals oxides, hydroxides and carbonates.
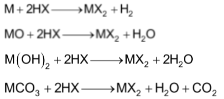
Properties
(1) All beryllium halides are essentially covalent and are soluble in organic solvents. They are hydroscopic and fume in air due to hydrolysis. On hydrolysis, they produce acidic solution.
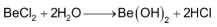
(2) The halides of all other alkaline earth metals are ionic. Their ionic character, however increases as the size of the metal ion increase.
(3) Except BeCl2 all other chlorides of group 2 form hydrates but their tendency to form hydrates decreases for eg – MgCl2.6H2O, CaCl2.6H2O.
(4) The hydrated chloride, bromides and iodides of Ca, Sr and Ba can be dehydrated on heating but those of Be and Mg undergo hydrolysis.
(5) BeF2 is very soluble in water due to the high hydration energy of the small Be+2 ion. The other fluorides (MgF2, CaF2, SrF2 and BaF2) are almost insoluble in water. Since on descending the group lattice energy decreases more rapidly than the hydration energy. Therefore whatever little solubility these fluorides have that increase down the group.
The chlorides, bromides and iodides of all other elements i.e. Mg, Ca, Sr, Ba are ionic have much lower melting points than the fluorides and are readily soluble in water. The solubility decreases some what with increasing atomic number.
(6) Except of BeCl2 and MgCl2, the other chlorides of alkaline earth metals impart characteristics colour to flame.
CaCl2 = Brick red colour
SrCl2 = Crimson colour
BaCl2 = Grassy green colour
Uses
(i) Calcium fluoride or fluorospar (CaF2) is by far the most important of all the fluorides of the alkaline earth metals since it is the only large scale source of fluorine.
(ii) CaCl2 is widely used for melting ice on roads, particularly in very cold countries because 30% eutectic mixture of CaCl2/ice freezes at 218 K as compared to NaCl /ice at 255K.
(iii) CaCl2 is also used as a desiccant (drying agent) in the laboratory.
(iv) Anhydrous MgCl2 is used in the electrolytic extraction of magnesium.
Solubility and Thermal stability of oxo salts
The salts containing one or more atoms of oxygen such as oxides, hydroxides, carbonates, bicarbonates, nitrites, nitrates, sulphates, oxalates and phosphates are called oxo salts.
Sulphates
The sulphates of alkaline earth metals (MSO4) are prepared by the action of sulphuric acid on metals, metals oxides, hydroxides and carbonates.
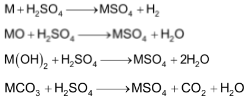
Properties of sulphates
The sulphates of alkaline earth metals are all white solids.
(a) Solubility
The solubility of the sulphates in water decreases down the groups i.e. Be > Mg > Ca > Sr > Ba.
Thus BeSO4 and MgSO4 are highly soluble, CaSO4 is sparingly soluble but the sulphates of Sr, Ba and Ra are virtually insoluble.
Reason
The magnitude of the lattice energy remains almost constant as the sulphate is so big that small increase in the size of the cation from Be to Ba does not make any difference. However the hydration energy decreases from Be+2 to Ba+2 appreciably as the size of the cation increase down the group. Hence, the solubilities of sulphates of alkaline earth metals decrease down the group mainly due to the decreasing hydration energies from Be+2 to Ba+2. The high solubility of BeSo4 and MgSO4 is due to high hydration energies due to smaller Be+2 and Mg+2 ions.
(b) Stability
The sulphates of alkaline earth metal decomposes on heating giving the oxides and SO3.
The temperature of decomposition of these sulpahtes increases as the basicity of the hydroxide of the corresponding metal increase down the group
Carbonates and Bicarbonates
Alkaline earth metal carbonates are obtained as white precipitates when.
(i) Calculated amount of carbon dioxide is passed through the solution of the alkaline metal hydroxides.
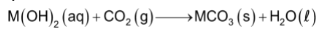
(ii) Sodium or ammonium carbonate is added to the solution of the alkaline earth metal salt such as CaCl2.
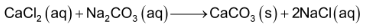
Properties
All carbonates are stable but beryllium carbonate is prone to hydrolysis. It contains the hydrated ion 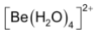 rather than Be+2 and hence is precipitated only in an atmosphere of CO2.
rather than Be+2 and hence is precipitated only in an atmosphere of CO2.

Solubility
The carbonates of magnesium and other alkali earth metals are sparingly soluble is water and their solubility decreases down the group from Be to Ba. For e.g MgCO3 is slightly soluble in water but BaCO3 is almost insoluble. The solubility can be explained by the reason same as for sulphates.
All the carbonates of alkaline earth metals are however, more soluble in the presence of CO2 due to the formation of corresponding bicarbonates.
For e.g.
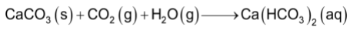
Stability
The carbonate of all alkaline earth metals decompose on heating to form the corresponding metal oxide and CO2.
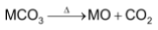
The temperature of decomposition, i.e thermal stability of these carbonates, however increase down the group from Be to Ba as the basicity of metal hydroxide increases from Be(OH)2 to Ba(OH)2.
The sulphates are stable to heat whereas the carbonates decompose to give MO and CO2. Thus BeCO3 is unstable and kept in the atmosphere of CO2 to prevent its decomposition.
BeCO3 MgCO3 CaCO3 SrCO3 BaCO3
<100°C 540°C 900°C 1290°C 1360°C
Bicarbonates
The bicarbonates of alkaline earth metals are prepared by passing CO2 through a suspension of metal carbonates in water.
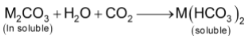
All the bicarbonates of alkaline earth metals are stable only in solution and have not been isolated in the pure state.
Nitrates
Alkaline earth metals nitrates are prepared in solution and can be crystallized as hydrated salts by the action of HNO3 on oxides hydroxides and carbonates.
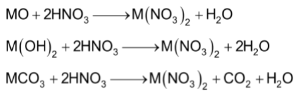
(M = Be, Mg, Ca, Sr or Ba)
Magnesium nitrate crystallizes as Mg(NO3)2.6H2O
White Ba(NO3)2 crystallises as unhydrous salt.
All nitrates on heating give the corresponding oxides.
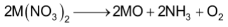
(M = Be, Mg, Ca, Sr or Ba)
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8









