Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
S and P Block Elements of Class 11
Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
The properties of lithium are quite different from the properties of other alkali metals. On the other hand, it shows greater resemblance with magnesium, which is diagonally opposite element of it.
The main reasons for the anomalous behaviour of lithium as compared to other alkali metals are
- The extremely small size of lithium atom and its ion.
- Greater polarizing power of lithium ion Li+, due to its small size which result in the covalent character in its compounds.
- Least electropositive character and highest ionization energy as compared to other alkali metals.
- Non availability of vacant d-orbitals in the valence shell.
The reason for resemblance of properties of lithium with magnesium is that these two elements have almost same polarizing power.
The following points illustrate the anomalous properties of lithium and its diagonal relationship with magnesium:
- The melting point and boiling point of lithium are comparatively high.
- Lithium is much harder than the other alkali metals. Magnesium is also hard metal.
- Lithium reacts with oxygen least readily to form normal oxide whereas other alkali metals form peroxides and superoxides.
-
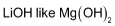 is weak base. Hydroxides of other alkali metals are strong bases.
is weak base. Hydroxides of other alkali metals are strong bases. - Due to their appreciable covalent nature, the halides and alkyls of lithum and magnesium are soluble in organic solvents.
-
Unlike elements of group 1 but like magnesium. Lithium forms nitride with nitrogen.

-
LiCl is deliquescent and crystallizes as a hydrate,
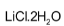 . Other alkali metals do not form hydrates.
. Other alkali metals do not form hydrates. 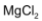 also forms hydrate,
also forms hydrate, 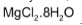 .
. - Unlike other alkali metals lithium reacts directly with carbon to form an ionic carbide. Magnesium also forms a similar carbide.
-
The carbonates, hydroxides and nitrates of lithium as well as magnesium decompose on heating.
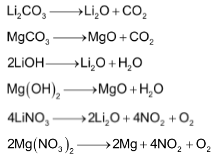
The corresponding salts of other alkali metals are stable towards heat. -
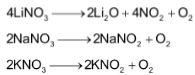
Lithium nitrate, on heating, decomposes to give lithium oxide,
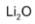 whereas other alkali metals nitrates decomposes to give the corresponding nitrite.
whereas other alkali metals nitrates decomposes to give the corresponding nitrite.
-
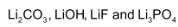 are the only alkali metal salts which are insoluble in water. The corresponding magnesium compounds are also insoluble in water.
are the only alkali metal salts which are insoluble in water. The corresponding magnesium compounds are also insoluble in water. - Hydrogen carbonates of both lithium and magnesium can not be isolated in solid state. Hydrogen carbonates of other alkali metals can be isolated in solid state.
Related Topics
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8









