Silicon
S and P Block Elements of Class 11
The silicon name is taken from Latin silver which means “flint”. The element is on second position in abundance in the earth’s crust after oxygen, was discovered by Berzelius in 1824.
The most common compound of silicon, SiO2 is the most abundant chemical compound in the earth’s crust.
Silicon is a crystalline semi – metal or metalloid. One of its forms is shiny, grey and very brittle. In another allotropic form silicon is a brown amorphous powder most familiar in “dirty” beach sand.
Germanium (Ge)
Like silicon, germanium is used in the manufacture of semi – conductor devices. But unlike silicon, it is rather rare. It was also predicted by Mendeleev in 1871 (ekasilicon) to fill out his periodic table and was discovered is 1886 Winkler. It is generally extracted from the by products of zinc – refining.
Tin (Sn)
It is one of the major elements along with copper used in bronze. It was named after the Etruscan god Tinia, the chemical symbols for it is taken from Latin Stannum . The metal is silvery white and very soft when pure. It has the look of freshly cut aluminium but the feel of lead. Polished tin is slightly bluish. It has been used for many years in the coating of steel cans for food because it is more resistant to corrosion than iron. It is chiefly used in solders. SnF2 is found in fluoride toothpastes.
Lead (Pb)
Its symbol came from ‘plumber’ word because since old plumbing was done with lead pipes. Although lead is not very common in earth’s crust, what is there is readily available and easy to refine. Its chief use today is in lead – acid storage batteries such as those used in automobiles.
In pure form it is too soft to be used for much else. Lead has a blue – white colour when first cut but quickly dulls on exposure to air forming Pb2O. Various isotopes of lead come at the end of the natural decay series of elements like uranium, thorium and actinium. These are Pb – 206, Pb – 207 and Pb – 208.
General Trends in Physical properties
|
Sr. No. |
Property |
Carbon |
Silicon |
Germanium |
Tin |
Lead |
|
1. |
Configuration |
[He] 2s22p2 |
[Ne]3s23p2 |
[Ar]4s24p2 |
[Kr]5s25p2 |
[Xe]6s26p2 |
|
2. |
Common oxidation state |
+4 |
+4 |
+4 |
+4, +2 |
+4, +2 |
|
3. |
Atomic radius (pm) |
77 |
117 |
122 |
140 |
175 |
|
4. |
First ionization energy (KJ/mol) |
1086 |
786 |
762 |
709 |
716 |
|
5. |
Electronegativity |
2.5 |
1.8 |
1.8 |
1.8 |
1.9 |
Catenation
A remarkable property of carbon is its ability to form compounds in which carbon atoms are linked to one another in chains or rings. This property of forming chains and rings is knows as catenation. On going down the group, tendency of catenation decreases.
C >> Si > Ge ≈ Sn >> Pb
Due to high tendency of catenation carbon forms bonds with other carbon atoms and forms so many compounds which are studied in organic chemistry.
Allotropy
A characteristic property of the elements of carbon family is that these show allotropy. Example: carbon has two important allotropic forms i.e. diamond and graphite. Allotropy is the existence of an element in two or more forms, which are significantly different in physical properties but have similar chemical properties. In diamond carbon is sp3 hybridised and has four tetrahedral bonds with adjacent carbon atoms. In graphite carbon is sp2 hybridised. The three cavalent bonds form hexagonal layers and fourth unhybridised p – electron of each carbon forms an extended delocalized π - bonding with carbon atoms of adjacent layers. Due to this free electron graphite is electric conductor while diamond is not. Also due to sliding property of graphite it has been using as a lubricant.
Chemical Properties of carbon family
Due to high ionization enthalpies M4+ ions of the group are not known hence the group elements mostly forms covalent compounds with a covalency of four.
Carbon differs distinctly from other elements of group 14 on the basis of its ability to form pπ - pπ multiple bonds to itself and to other elements like nitrogen and oxygen.
The chemical characteristics of the group 14 elements are discussed below:
1. Hydrides
Carbon forms large number of cyclic and acyclic hydrides known as hydrocarbons. These have general formulas of type CnH2n+2 (alkanes), CnH2n(alkenes), C2H2n−2 (alkynes) etc.
Silicon and germanium also forms few hydrides of formula MnH2n+2 (where M = Si, n = 1 to 8 or
M = Ge, n = 1 to 5). These are known as Silanes and Germanes respectively. Only one hydride of tin, namely monostannane (SnH4) is known.
Thermal stability of these hydrides decreases down the group. The reducing characters of these hydrides increases on going down the group.
2. Oxides
The group forms two types of oxides, monoxides and dioxides.
(i) Monoxides
All except Si forms monoxides of general formula MO, such as CO, GeO, SnO and PbO. CO is only neutral otherwise all are basic in nature.
Structure of CO
The molecular orbital configuration of CO is which suggests the presence of triple bond between carbon and oxygen atoms. However, CO molecule is considered to be a resonance hybrid of the following structures:
CO, is colourless toxic gas and it forms a number of co – ordination compounds with transition metals e.g, Ni(CO)4, Fe(CO)5 and Cr(CO)6. Such compounds are called as organometallic compounds because carbon is bonded to metals.
(ii) Dioxides
All the elements of groups 14 forms dioxides of general formula MO2, such as CO2, such as CO2, SiO2, GeO2, SnO2 and PbO2.
(a) Among these oxides CO2 and SiO2 are acidic whereas GeO2, SnO2 are amphoteric in nature and PbO2 behaves as base.
SnO2, as it is amphoteric reacts with acid as well as base.
(b) PbO2 powerful oxidizing. It readily dissolves in acids forming Pb(II) salts with the liberation of oxygen.
(c) CO2 differs very much from other dioxides. CO2 is gas at room temperature. SiO2 is solid at room temperature. CO2 is monomeric, nonpolar and linear molecule while SiO2 exists in 3-D structure.
(iii) Carbon suboxide (C3O2)
In addition to CO an CO2, C3O2 in also formed by carbon.
It is a foul smelling gas with b.p. 6°C. It has reaction with H2O or HCl.
3. Halides
There forms MX4 type halides and except carbon others also forms MX2 type. The stability of MX2, dihalide increases on moving down the group.
CX2 << SiX2 << GeX2 << SnX2 << PbX2
Except CX4 all other tetrahalides can be hydrolysed, due to presence of vacant d – orbitals. Due to this reason only tetrahalides of all the elements in group 14 except carbon can react with halogen acids. e.g.
Ionic character and thermal stability decreases with the increase in size of halide ion.
Tin(II) chloride is obtained by dissolving tin in conc. HCl; when the solution is cooled, crystals of tin (II) chloride dehydrate SnCl2.2H2O separate out.
Anhydrous SnCl2 is prepared by heating tin in a current of HCl vapour. SnCl2 is used as a reducing agent in acid solution.
Lead (II) halides are formed by adding halide ions to a soluble lead salt.
Pb(II) halides are colourless solids excepts PbI2, which is yellow. They are sparingly soluble in water. The formation of PbCl2 or PbI2 serves as a test for the detection of Pb2+ in qualitative analysis.
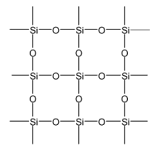
Silica (SiO2)
Silicon is unable to form pπ − pπ bond with oxygen atom due to its relatively large size. Thus it satisfies its all four valency with four oxygen atoms and constitutes three - dimensional network. In this structure each oxygen atom is shared by two silicon atoms. Three crystalline modification of SiO2 are quartz, cristobalite and tridymite of which quartz and cristobalite are important.
Quartz (rock crystal) is the purest form of silica. It is used in preparation of costly glasses and lenses. It is also used as piezoelectric material (crystal oscillators and transducers).
Several amorphous forms of silica such as silica gel and fumed silica are known. Silica gel in made by acidification of sodium silicate and when dehydrates, is extensively used as a drying agent in chromatographic and catalyst support.
Structure of Silica
|
|
Artificially silica can be obtained by following methods.
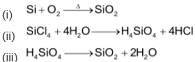
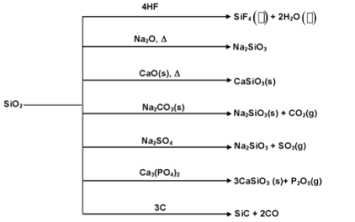
Properties of Silica
Pure silica is colourless but sand is brownish or yellowish due to presence of impurities of iron oxide.
Silicates and Silicones
(A) Silicates
This is the general term applied for the solids with silicon – oxygen bonds. Some of the silicate minerals are quartz, asbestos (CaMgSi2O6), feldspar (KAlSi3O8), mica [KAl2(Si3AlO10) (OH)2] and zeolites (Na2Al2SiO8.xH2O).
The solids contain silicate ion (SiO4)4- as the basic structural unit. The silicate ion is tetrahedral in structure and when the one or more oxygen atoms between such tetrahedrons, a complex structure arise.
The silicates may be classified in to chain silicates, ring silicates, cyclic silicates, sheet silicates, three – dimensional silicates depends on the way in which the (SiO4)4− tetrahedral units are linked together.
(b) Silicones
Silicones are polymeric compounds containing repeated R2SiO units. The name is given silicone because their empirical formula is analogous to that of ketones (R2CO).
Silicones are chemically inert, water repelling nature, heat resistance and having good electrical insulting properties. They are used as sealants, greases, electrical insulators and for water proofing of fabrics.
Commercial silicon polymers are usually methyl derivatives and to a lesser extent phenyl derivatives. They are prepared by the hydrolysis of R2SiCl2(R = Me or Ph).
The starting alkyl substituted chlorosilanes are obtained by direct reaction of RCl with silicon in the presence of metallic copper as a catalyst.
It is interesting to note that hydrolysis of alkyl trichlorosilanes, RSiCl3 gives cross linked polymers instead of chain polymers.
Zeolites
Zeolites are microporus aluminosilicates of general formula 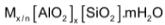 and may be considered as open structure of silica in which aluminium has been substituted in a fraction of x/(x+y) of the tetrahedral sites. The aluminosilicates framework is constituted by negative charge and exchangeable cations of valence n. the void space which is approximately 50% of the volume is occupied by m molecules of water in the unitcell.
and may be considered as open structure of silica in which aluminium has been substituted in a fraction of x/(x+y) of the tetrahedral sites. The aluminosilicates framework is constituted by negative charge and exchangeable cations of valence n. the void space which is approximately 50% of the volume is occupied by m molecules of water in the unitcell.
Extraction of Tin
The chief ore of tin is cassiterite (SnO2) which on reduction with carbon produces tin. This ore contains about 10% of tin. It is extracted in following manner:
(i) The ore is crushed and washed with water to remove lighter impurities.
(ii) It is roasted than to remove impurities such as arsenic and sulphur as volatile oxides.
(iii) The roasted ore is heated with coal in a reverberatory furnace at 1500 K.
The crude tin obtained as above is contaminated with iron, lead and other metals. Its is therefore remelted on an inclined surface. Tin has a lower melting point hence melt first and flows down leaving other less fusible metals.
Molten tin is finally stirred with green poles of wood in contact of air. In this process any remaining metals impurities are oxidized forming a scum which rises to the surface and is removed. Thus process is called poling.
Extraction of Lead
The ores of lead are:
(i) Galena, PbS
(ii)Cerussite, PbCO3
(iii) Anglesite, PbSO4
The chief ore is galena (PbS). The ore is first concentrated by froth – floatation and then roasted in a limited supply of air to give PbO which is reduced to the metal by heating with coke and limestone (flux) in a blast furnace.
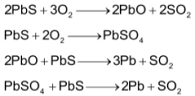
In another way, the mixed sulphides (PbS + ZnS) are roasted to obtain oxides. The mixed oxides are reduced to their respective metals with coke by heating in blast furnace.
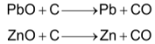
Molten lead is trapped from the bottom of the furnace. Zinc vapours which come out from the top of the furnace are condensed.
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8