Chemical Properties
S and P Block Elements of Class 11
Chemical Properties
The alkali metals are highly reactive metals and the reactivity increases down the group. The reactivity is due to-
(a) low value of first ionization energy
(b) large size
(c) low heat of atomization
(i) Reaction with Oxygen
The alkali metals tarnish in air due to the formation of an oxide or hydroxide on the surface. Alkali metals when burnt in air form different kinds of oxides. For eg, the alkali metals on reaction with limited quantity of oxygen form normal oxides of formula, M2O

When heated with excess of air, lithium forms normal oxide, 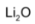 sodium forms peroxide
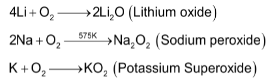
sodium forms peroxide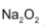 , whereas potassium rubidium and caesium form superoxides having general formula
, whereas potassium rubidium and caesium form superoxides having general formula 
Thus the reactivity of alkali metals with oxygen increases down the group. Further, the increasing stability of peroxide or superoxide, as the size of the metal ion increases is due to the stabilization of larger anions by larger cation through higher lattice energies.
Due to small size, has a strong positive field around it which attracts the negative charge so strongly that it doe not permit the oxide anion to combine with another oxygen to form peroxide ion,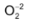 . On the other hand, ion because of its large size than Li+ ion has comparatively weaker positive field around it which cannot prevent ion to combine with another oxygen to form peroxide ion
. On the other hand, ion because of its large size than Li+ ion has comparatively weaker positive field around it which cannot prevent ion to combine with another oxygen to form peroxide ion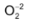 . The larger
. The larger 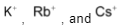 ions have still weaker positive field around them which cannot prevent even peroxide ion,
ions have still weaker positive field around them which cannot prevent even peroxide ion, 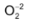 to combine with another oxygen atom to form superoxide
to combine with another oxygen atom to form superoxide 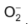 .
.
(ii) Reaction with Hydrogen
Alkali metals react with dry hydrogen at about 673K to form colourless crystalline hydrides. All the alkali metal hydrides are ionic solids with high melting points.
(M = Li, Na, K, Rb or Cs)
Some important features of hydrides are
(a) The stability of hydrides decrease from Li to Cs. It is because of the fact that M-H bond becomes weaker due to increase in the size of alkali metals down the group.
(b) These hydrides react with water to form corresponding hydroxides and hydrogen gas.
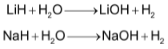
(c) These hydrides are strong reducing agents and their reducing nature increases down the group.
Alkali metals also form complex hydrides such as  and
and  which are good reducing agents.
which are good reducing agents.
(d) All these hydrides react with proton donors such as water, alcohols, gaseous ammonia and alkynes liberating gas.
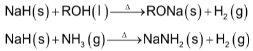
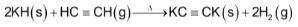
The order of reactivity of the alkali metals towards hydrogen decreases as we move down the group from Li to Cs. This is due to the reason that the lattice energies of these hydrides decreases progressively as the size of the metal cation increases and thus the stability of these hydrides decreases from LiH to CsH.
(iii) Reaction with water
The alkali metals are known to have large negative reduction potential values. As a result they can act as better reducing agents as compared to hydrogen. Hence, alkali metals react with water and other compounds containing acidic hydrogen atoms such as hydrogen halides (HX) and acetylene (C2H2) and liberate H2 gas
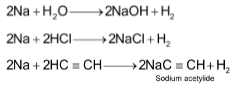
The reaction becomes more and more violent as we move down the group. Thus, Lithium reacts gently, sodium melts on the surface of water and the molten metal moves around vigorously and may sometimes catch fire. Potassium melts and always catches fire and so are Rb and Cs.
(iv) Reaction with halogens
Alkali metals react vigorously with halogens to form metal halides of general formula MX, which are ionic crystalline solids.
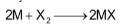
M = Li, Na, K, Rb or Cs and
X = F, Cl, Br or I
Reactivity of alkali metals with particular halogens increases from Li to Cs. On the other hand, reactivity of halogens decreases from 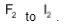 .
.
(v) Solubility in liquid ammonia
All alkali metal dissolve in liquid ammonia giving deep blue solutions which are conducting in nature. These solutions contain ammoniated cations and ammoniated electrons as shown below:
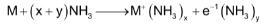
The blue colour of the solution is considered to be due to ammoniated electrons which absorb energy corresponding to red region of the visible light for the their excitation to higher energy levels. The transmitted light is blue which imparts blue colour to the solutions. The electrical conductivity of the solution is due to both ammoniated cations and ammoniated electrons. The blue solution on standing slowly liberates hydrogen resulting in formation of amide :
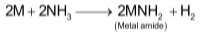
At concentrations above 3M, the solutions of alkali metals in liquid ammonia are copper-bronze coloured. These solutions contains clusters of metal ions and hence possess metallic lusture. The blue coloured solutions are paramagnetic due to presence of large number of unpaired electrons, but bronze solutions are diamagnetic due to formation of electron clusters in which ammoniated electrons with opposite spin group together
These solutions are stronger reducing agents than hydrogen and hence will react with water to liberate hydrogen.
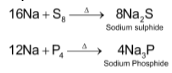
(vi) Reaction with sulphur and phosphorus
Alkali metals react with sulphur and phosphorus on heating to form sulphides and phosphides respectively.
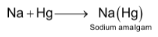
(vii) Reaction with Mercury
Alkali metals combine with mercury to form amalgams. The reactions is highly exothermic in nature
- Introduction
- Physical Properties
- Chemical Properties
- General Characteristic Of The Compounds Of The Alkali Metals
- Anomalous Behaviour Of Lithium And Its Diagonal Relationship With Magnesium
- Difficulties Encountered During Extraction Of Alkali Metals
- Sodium
- Compounds Of Alkali Metals
- Alkaline Earth Metals
- Extraction Of Lithium
- Chemical Properties Of Group II elements
- Anamalous Behaviours Of Beryllium
- Manufacture Of Cement
- Silicon
- Exercise 1
- Exercise 2
- Exercise 4
- Exercise 5
- Exercise 6
- Exercise 7
- Exercise 8









