Amino Acid
Molecules of Cell of Class 11
Amino acids are the basic units from which proteins are made. Over 170 amino acids are currently known to occur in cells and tissues. Of these, only 20 are commonly found in proteins.
.png)
Fig. Common amino acids found in proteins :
Only the R groups are shown. The box represents the constant part of the molecule.
In an amino acid, there is a central carbon atom, to which is always attached an acidic carboxyl group, –COOH, a basic amino group, –NH2 and a hydrogen atom. The fourth position is the only variable part of the molecule. The group here is known as the R group. This group gives each amino acid its uniqueness. The simplest amino acid, is glycine, where R is simply hydrogen. When R is –CH3, the amino acid alanine is formed.
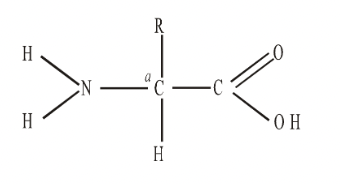
Fig. General Formula of an amino acid
Amino acids contain both an acid and a basic part and are described as amphoteric. They exist mainly as ions and can carry both a positive charge on the basic part and a negative charge on the acid part. Such ions are described as dipolar and are called zwitterions.
Amino acids combine to form proteins. They are joined together by a type of bond known as a peptide bond.
Peptide bond is formed when a water molecule is eliminated during a reaction between the amino group of one amino acid and the carboxyl group of another and the compound formed is a dipeptide. It possesses a free amino group at one end, and a free carboxyl group at the other. This enables further combination between the dipeptide and other amino acids. If many amino acids are joined together in this way, a polypeptide is formed.
Once these bonds have been formed, the protein typically folds into a particular shape as a result of four other types of bond, namely ionic bonds, disulphide bonds, hydrogen bonds, and hydrophobic interactions.
Acidic and basic R groups exist in an ionised (charged) state at certain pHs. Acidic R groups are negatively charged and basic R groups are positively charged. They can therefore be attracted to each other, forming ionic bonds. In an aqueous environment this bond is much weaker than a covalent bond and can be broken by changing the pH of the medium. For example, adding acid to milk makes it curdle because the ionic bonds in casein (milk protein) are broken and the protein ceases to be soluble.
The amino acid cysteine contains a sulphydryl group, –SH, in its R group. If two molecules of cysteine line up alongside each other, neighbouring sulphydryl groups can be oxidised and form a disulphide bond. Disulphide bonds may be formed between different chains of amino acids or between different parts of the same chain in which they and make the molecule fold into a particular shape. They are strong and not easily broken. Some R groups are non-polar and therefore hydrophobic, such as those on the amino acids tyrosine and valine. If a polypeptide chain contains a number of these groups and is in an aqueous environment, the chain will tend to fold so that the maximum number of hydrophobic groups come into close contact and exclude water. This is how many globular proteins fold up. The hydrophobic groups tend to point inwards towards the centre of the roughly spherical molecule while the hydrophilic groups face outwards into the aqueous environment, making the protein soluble.
Plants are able to make all the amino acids they require from simpler substances. However, animals are unable to synthesise all that they need, and therefore must obtain some ‘ready- made’ amino acids directly from their diet. These are termed essential amino acids. Animals can make the other amino acids they require from these.
A small number of rare amino acids occur in the proteins of organisms which are made from some of the common amino acids. There is no DNA code for the rare amino acids, and they are made from their parent amino acids after they have been incorporated into a protein. For example, hydroxyproline is made from proline, and is found in the protein collagen; hydroxylysine is made from lysine, and is also found in collagen.
Non-protein amino acids over 150 are known to occur, either free or in a combined form in cells, but never in proteins. Ornithine and Citrulline take part in urea cycle.
Homoserine is intermediate in synthesis of cysteine, methionine and threonine.
Diaminopimelic acid is intermediate in synthesis of lysine.
D-Alanine and D-Glutamic acid are component of peptidoglycan of bacterial wall.
A number of antibiotics contain amino acid analogues that prevent protein synthesis, e.g. D-Phenylalanine in Gramicidin-S, Actinomycin-D, an inhibitor of RNA synthesis, contains D- Valine.
GABA (aminobutyric acid) is virtually unique to the nervous system. It is an inhibitory neurotransmitter, important in the brain.
- Introduction of Cell Molecule
- Carbohydrates
- Structural Polysaccharides
- Sugar Part in Carbohydrates
- Storage Of Polysaccharides
- Lipids
- Constituents Of Lipids
- Types Of Lipids
- Amino Acid
- Protein
- Classification of Proteins
- Structure Of Proteins
- Denaturation and renaturation of proteins
- Nucleotides
- Structures of Nucleotides
- Formation of Nucleotides
- Vitamines of Nucleotides
- Nucleic Acid
- Minerals
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









