Carbohydrates
Molecules of Cell of Class 11
Carbohydrates are substances which contain the elements carbon, hydrogen and oxygen and have the general formula Cx(H2O)y, where x and y are variable numbers; their name (hydrate of carbon) is derived from the fact that hydrogen and oxygen are present in the same proportions as in ‘water’ namely two hydrogen atoms to one oxygen atom.
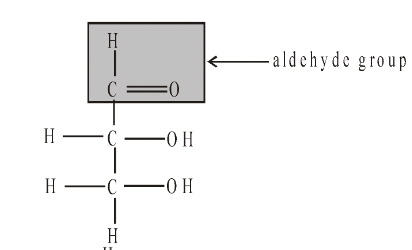
They are aldehydes or ketones and all contain several hydroxyl groups. Their chemistry is determined by these groups. e.g. aldehydes are very easily oxidised and hence are powerful reducing agents.
.png)
Glyceraldehyde Dihydroxyacetone
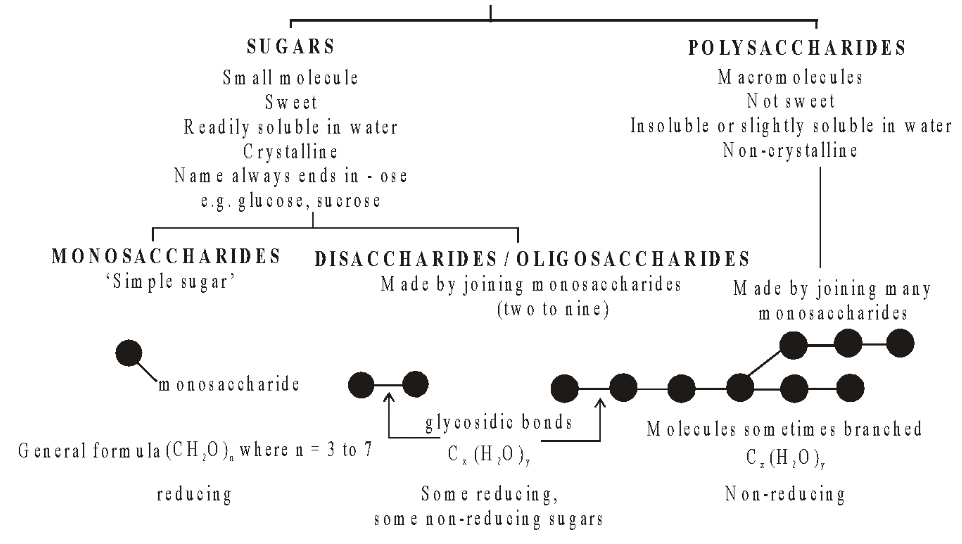
In monosaccharides, all the carbon atoms except one have a hydroxyl group attached. The remaining carbon atom is either part of an aldehyde group, in which case the monosaccharide is called an aldose or aldo sugar, or is part of a keto group, when it is called a ketose or keto sugar. Thus all monosaccharides are aldoses or ketoses. The two simplest monosaccharides are the trioses-glyceraldehyde and dihydroxyacetone. Glyceraldehyde has an aldehyde group and dihydroxyacetone has a keto group. In general, aldoses, such as ribose and glucose, are more common than ketoses, such as ribulose and fructose. Carbohydrates are divided into three main classes, Monosaccharides, Oligosaccharides and Polysaccharides.
- Introduction of Cell Molecule
- Carbohydrates
- Structural Polysaccharides
- Sugar Part in Carbohydrates
- Storage Of Polysaccharides
- Lipids
- Constituents Of Lipids
- Types Of Lipids
- Amino Acid
- Protein
- Classification of Proteins
- Structure Of Proteins
- Denaturation and renaturation of proteins
- Nucleotides
- Structures of Nucleotides
- Formation of Nucleotides
- Vitamines of Nucleotides
- Nucleic Acid
- Minerals
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









