
Nucleic Acid
Molecules of Cell of Class 11
Nucleic acids were first isolated by Swiss physician Friedrich Miescher (1868-1869) from nuclei of pus cells named as nuclein.
Altman (1889) renamed nuclein as nucleic acid.
Buhrem (1938) differentiated two types of nucleic acids, DNA and RNA. Usually both of them occur in a cell with the exception of viruses which have only one of them.
Nucleic acids form the genetic material of all living organisms including the simplest virus.
Nucleic acids are made up of units called nucleotides which are arranged to form extremely long molecules known as polynucleotides.
DNA or Deoxyribonucleic Acid
DNA or deoxyribonucleic acid is helically twisted double chain mixed polymer of deoxyribonucleotides which is not only the largest macromolecule but also represents genetic material of organisms.
DNA is, molecular basis of heredity occurs inside nucleus, mitochondria and plastids. DNA present inside nucleus is called nuclear DNA. The one found in mitochondria and plastids is called organelle DNA.
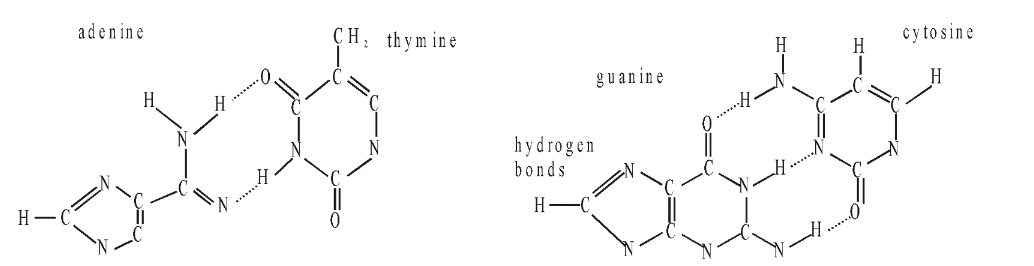
Fig. Adenine-thymine and guanine-cytosine base pairs
Watson and Crick published their model in 1953 in the journal Nature and, together with Maurice Wilkins, were awarded the Nobel Prize for their work in 1962, the same year that Kendrew and Perutz received Nobel prizes for their work on the three-dimensional structure of proteins, also based on X-ray crystallography.
Watson and Crick showed that DNA consists of two polynucleotide chains. Each chain forms a right-handed helical spiral and the two chains coil around each other to form a double helix.
The chains run in opposite directions, that is are antiparallel, end of one strand faces the end of other strand.
Each chain has a sugar-phosphate backbone with bases which project at right-angles and hydrogen bond with the bases of the opposite chain across the double helix.
The width between the two backbones is constant (20 Å) and equal to the width of a base pair, that is the width of a purine plus a pyrimidine. Two purines would be too large and two pyrimidines too small, to span the gap between the two chains.
Three hydrogen bonds occur between cytosine and guanine 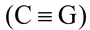 (C G)at positions 1–1, 2–6 and 6–2. There are two such hydrogen bonds between adenine and thymine (A=T) which are formed at positions 1–3 and 6–4. Hydrogen bonds occur between hydrogen of one base and oxygen or nitrogen of the other base.
(C G)at positions 1–1, 2–6 and 6–2. There are two such hydrogen bonds between adenine and thymine (A=T) which are formed at positions 1–3 and 6–4. Hydrogen bonds occur between hydrogen of one base and oxygen or nitrogen of the other base.
Along the axis of the molecule the base pairs are 0.34 nm apart, accounting for the regularity indicated by X-ray diffraction. A complete turn of the double helix comprises 3, 4 nm or ten base pairs.
There is no restriction on the sequence of bases in one chain, but because of the rules of base pairing, the sequence in one chain determines that in the other. The two chains are thus said to be complementary.
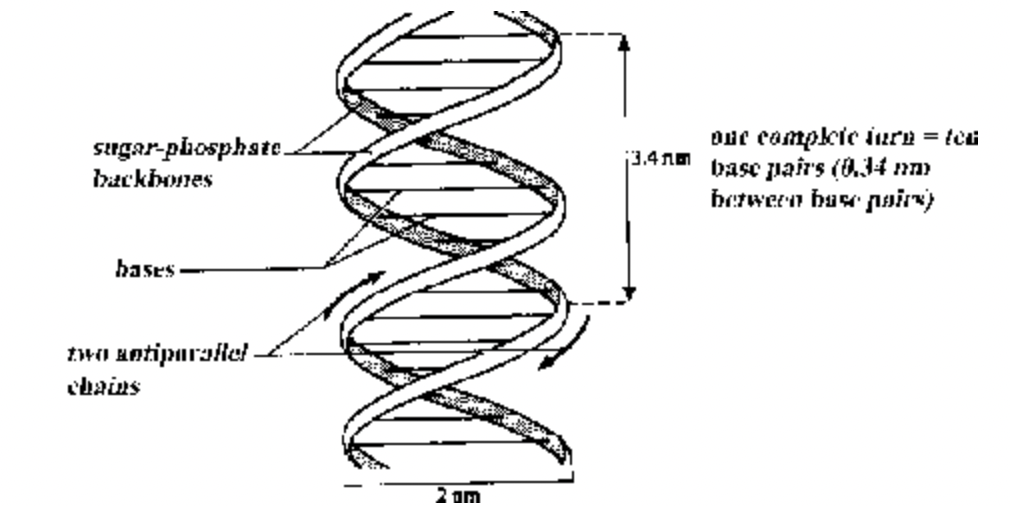
Fig. Diagrammatic structure of DNA
Both the strands of DNA do not take part in controlling heredity and metabolism. Only one of them does so. The DNA strand which functions as a template for RNA synthesis is known as template strand, minus (–) strand or antisense strand. Its complementary strand is named as nontemplate strand, plus (+) strand, sense strand or coding strand (because by convention DNA) genetic code is written according to its sequence. This is despite the fact that it has no role in transcription.
(5‛) GCATTCGGCTAGTAAC (3‛) DNA Nontemplate, Sense, Coding or (+) Strand
(3‛) CGTAAGCCGATCATTG (5‛) DNA template, Antisense or (–) Strand
(5‛) GCAUUCGGUAGUAAC (3‛) RNA Transcript
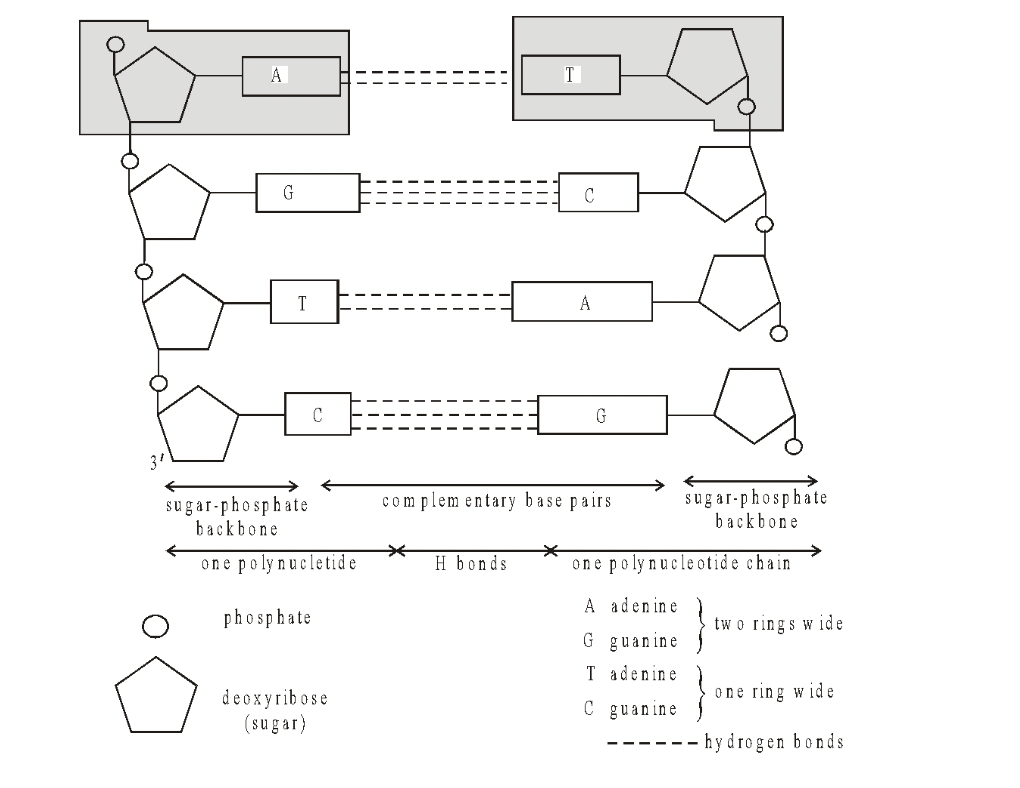
Fig. DNA—Diagrammatic structure of straightened chains
DNA is helically coiled long double strand. In many procaryotes, the two ends get connected to form circular DNA. Circular DNA is naked, that is, without association with histone proteins; though polyamines do occur.
In linear DNA, the two ends are free. It is found in eucaryotic nuclei where it is associated with histone proteins. Linear DNA, without association with histones, also occurs in some procaryotes, e.g. Mycoplasma. In semi-autonomous cell organelles (mitochondria, plastids) DNA is circular, less commonly linear. It is always naked. Chargaff’s Rules : Chargaff (1950) made observations on the base and other contents of DNA. These observations or generalizations are called Chargaff’s rule or base pairing rules.
(i) Purine and pyrimidine base pairs are in equal amount, that is, adenine + guanine = thymine + cytosine.
(ii) Molar amount of purine adenine is always equal to the molar amount of thymine. Similarly, guanine is equalled by cytosine.
(iii) Sugar deoxyribose and phosphate occur in equimolar proportions.
(iv) The ratio A + T/G + C is constant for a species. It can be used to identify the source of DNA. Denaturation (=Melting). The hydrogen bonds between the nitrogen bases of complementary DNA strands can break due to high temperature, low or high pH. The phenomenon is called denaturation or melting. Since an A–T base pair has only two hydrogen bonds, the area rich in A–T base pairs can undergo easy denaturation. It is known as low melting area. The area rich in G–C base pairs is comparatively more stable because three hydrogen bonds and form duplex. The phenomenon is called renaturation. DNA duplex possesses areas where sequence of nucleotides is the same but opposite in the two strands, e.g.
–T–T–A–A–C–G–T–T–A–A–
–A–A–T–T–G–C–A–A–T–T
These areas are called palindromes or palindromic regions. Regions connected with transcription of ribosomal RNA are often palindromic. The exact significance of this sort of arrangement is not known.
Repetitive DNA consists of a sequence of nitrogen base repeated several times in tandem. Moderately repetitive DNA is found in telomeres, centromeres and transposon ends. Highly repetitive DNA is present in precentromeric regions, heterochromatic regions of Y-chromosome and satellite regions.
Genetic Information. The arrangement of nitrogen bases of DNA (and its product mRNA) determines the sequence of amino acid groups in polypeptides or proteins formed over ribosomes. One amino acid is sepcified by the sequence of three adjacent nitrogen bases called as codon. The segment of DNA which determines th synthesis of a complete polypeptide is known as cistron. In procaryotes, a cistron has a continuous codon sequence from beginning to end. In eucaryotes a cistron contains regions which do not code for amino acids and are called introns. The coding parts are known as exons. Cistrons having introns are called split genes.
Forms of DNA.
The common DNA is B-DNA. It is stable right handed double helix with a width of 20Å with about 10 base pairs per turn of spiral that is 34Å long. Base pairs are separated by 3.4 Å.
Other forms of DNA are unstable.
In A-DNA, the diameter is more, base pairs per turn are 11 while rise per base pair is 2.3 Å.
In C-DNA per turn of spiral has 9 base pairs.
It is 8 base pairs in D-DNA.
7.5 base pairs in E-DNA. All these are right handed DNAs.
Z-DNA is left handed. Its back bone is zig-zig, thickness is 18Å while distance per turn of spiral is 45Å. There are 12 base pairs per turn of spiral. Rise per base pair is 3.8 Å.
RNA or Ribonucleic Acid
RNA exists as a single stranded molecule in all living cells. It differs from DNA in possessing the pentose sugar ribose
instead of deoxyribose and the pyrimidine uracil instead of thymine.
Three types of RNA are found in cells, which are all involved in the synthesis of protein molecules. These are messenger
RNA (mRNA), transfer RNA (RNA) and ribosomal RNA (rRNA). All three types are synthesised directly on DNA, and
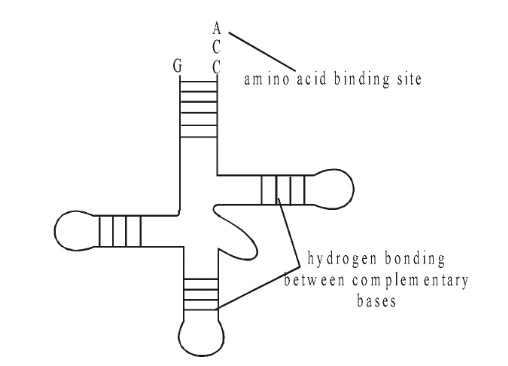
the amount of RNA in each cell is directly related to the amount of protein synthesis. Fig. tRNA
Messenger RNA
The base sequence of mRNA is a complementary copy of the DNa strand being copied and varies in length according to the length of the polypeptide chain for which it codes.
Most mRNA exists within the cell for a short time. In the case of bacteria this may be a matter of
minutes whereas in developing red blood cells the mRNA may continue to produce haemoglobin for several days.
Ribosomal RNA
Ribosomal RNA makes up approximately 80% of the total RNA of the cell.
It is synthesised by genes present on the DNA of several chromosomes found within a region of the nucleolus known as the nucleolar organiser.
The base sequence of rRNA is similar in all organisms from bacteria to higher plants and animals. It is found in the cytoplasm where it is associated with protein molecules which together
form the cell organelles known as ribosomes.
Ribosomes are the sites of protein synthesis. Here the mRNA ‘code’ is translated into a sequence of amino acids in a polypeptide chain.
Transfer RNA
The existence of transfer RNA (tRNA) was postulated by Crick and demonstrated by Hoagland in 1955.
Each amino acid has its own family of tRNA molecules.
RNA transfers amino acids present in the cytoplasm to the ribosome. Consequently it acts as an intermediate molecule between the triplet code of mRNA and the amino acid sequence of the polypeptide chain.
It makes up about 15% of the total RNA of the cell and, having on average 80 nucleotides per molecule, it is the smallest of all the RNAs.
There are more than 20 different tRNA molecules in a given cell (60 have so far been identified) carrying specific amino acids. All tRNA molecules have the same basic structure.
The 5’end of the tRNA always ends in the base guanine whilst the 3’end always ends in the base sequence of CCA.
The base sequence of the rest of the molecule is variable and may include some ‘unusual bases’ such as inosine (I) and pseudouracil (y).
The triplet base sequence at the anticodon is directly related to the amino acid carried by that tRNA molecule.
Each amino acid is attached to its specific tRNA by its own form of the enzyme aminoacyl-tRNA synthetase. This produces an amino acid-tRNA complex known as aminoacyl-tRNA with
sufficient energy in the bond between the final A nucleotide of CCA and the amino acid to later form a peptide bond with the adjacent amino acid. In this way a polypeptide chain is synthesised.
