Sugar Part in Carbohydrates
Molecules of Cell of Class 11
MONOSACCHARIDES
Monosaccharides are single sugar units. Their general formula is (CH2O)n. They are classified according to the number of carbon atoms as trioses (3C), tetroses (4C), pentoses (5C),
hexoses (6C) and heptoses (7C). Of these, pentoses and hexoses are the most common.
Monosaccharides are important as energy sources and as building blocks for the synthesis of larger molecules.
Types :
1. Trioses (C3H6O3) e.g. Glyceraldehyde, Dihydroxyacetone. Intermediates in respiration (glycolysis), photosynthesis (dark reactions) and other kinds of carbohydrate metabolism.
2. Tetroses (C4H8O4) e.g. Erythrose, Threose, Erythrulose
Erythrose is an intermediate of respiratory and photosynthetic pathways and raw material for the synthesis of lignin, anthocyanins and some amino acids (like tyrosine and phenylalanine).
3. Pentoses (C5H15O5) e.g. ribose, deoxyribose (C5H10O4), xylose, arabinose, ribulose.
Synthesis of nucleic acid; ribose is a constituent of RNA, deoxyribose of DNA.
Synthesis of some coenzymes e.g. ribose used in the synthesis of NAD and NADP.
Ribulose biphosphate is the CO2 acceptor in photosynthesis.
Arabinose and xylose form polymers arabans and xylans which are constituents of hemicellulose.
4. Hexoses (C6H12O6) e.g. glucose, fructose, galactose, mannose.
White, crystalline and sweet substances.
Glucose is blood sugar, a common respiratory substrate. Also called grape sugar, corn sugar and dextrose.
Fructose is sweetest naturally occuring sugar. Nectar and honey contain L form of fructose called Levulose. Also called fruit sugar.
5. Heptoses (C7H14O7) e.g. Sedoheptulose, Glucoheptose, Galactoheptose.
Sedoheptulose is intermediate of respiratory and photosynthetic pathways.
Open Chain and Ring Forms
Pentoses and hexoses have an ‘open chain’ or ring structure. The open chain form can be straight, but because of the bond angles between carbon atoms it is possible for sugars with five and six carbon atoms to bend and form stable ring structures.
In hexoses like glucose, the first carbon atom combines with the oxygen atom on carbon atom number five to give a six-membered ring (pyranose form). Oxygen is part of the ring and carbon atom number 6, sticks up out of the ring.
In pentoses, the first carbon atom joins with the oxygen atom on the fourth carbon atom to give a five membered ring. (furanose form) The ring structures of pentoses and hexoses are more stable and more common, with only a small proportion of the molecules existing in the open chain form at any one time. The ring structure is the form used to make disaccharides and polysaccharides.
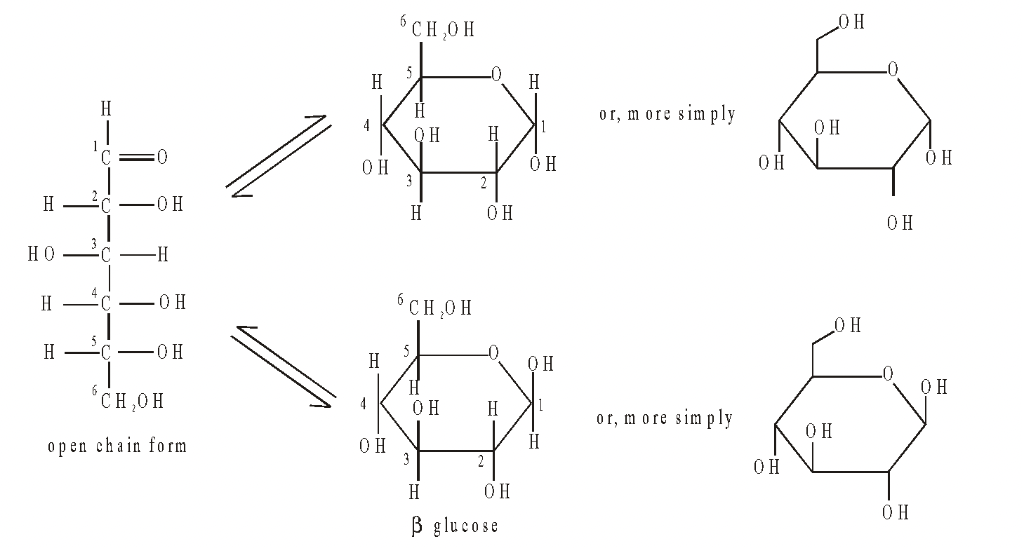
Fig. Structure of the open chain and and ring forms of glucose
Glucose can exist in two possible ring forms, known as the alpha and beta forms. The hydroxyl group on carbon atom 1 can project below the ring ( glucose) or above the ring ( glucose).
OLIGOSACCHARIDES
Small sized polymers of monosaccharides having 2 simple sugars, occasionally upto 9-10. They are formed by condensation of two or more monosaccharides,Oligosaccharides are compound carbohydrates like polysaccharides but are of comparatively low molecular weight.
They are soluble in water, sweet to taste and crystalline in nature. The successive monosaccharide residues are linked by means of glycosidic bonds (–C–O–C–).
Smaller oligosaccharides are unbranched. The larger ones can be branched. Such oligosaccharides occur as components of glycoproteins, glycophorins and glycolipids.
They take part in recognition and antigenic properties.
Depending upon the number of monosaccharide residues present, oligosaccharides are called disaccharides, trisaccharides, tetrasaccharides, pentasaccharides and hexasaccharides.
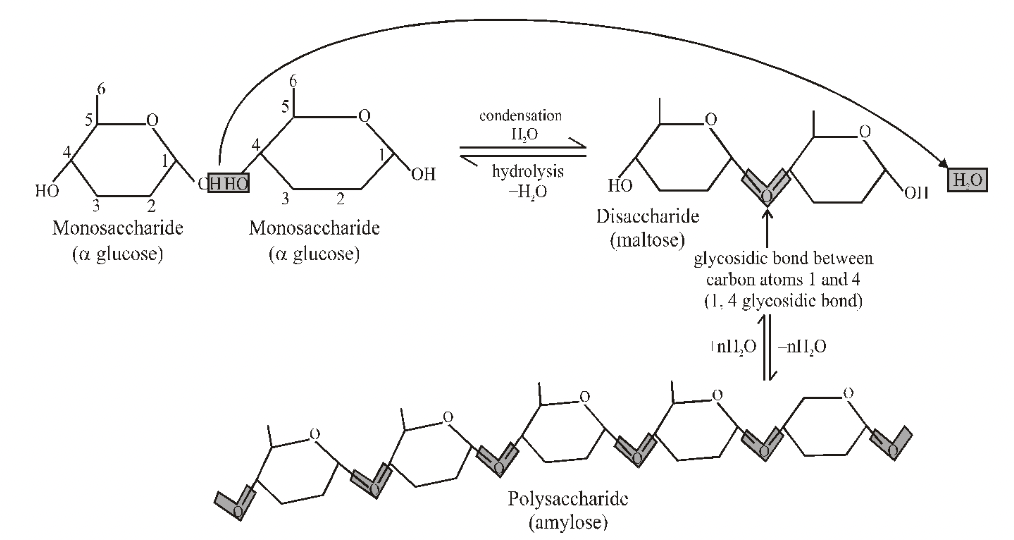
Disaccharides
Formed when two monosaccharides, usually hexoses, combine by means of a chemical reaction known as condensation means removal of water.

The bond formed between two monosaccharides is called a glycosidic bond.
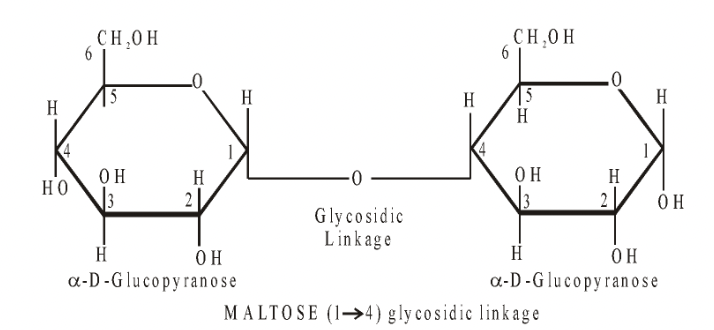
The most common disaccharides are maltose, lactose and sucrose :
Maltose = Glucose + Glucose
Occurs mainly as a breakdown product during digestion of starch by enzymes called amylases.
This commonly occurs in animals and in germinating seeds.
Lactose = Glucose + Galactose
Milk sugar, is found exclusively in milk and is an important energy source for young mammals. It can only be digested slowly, so gives a slow steady release of energy.
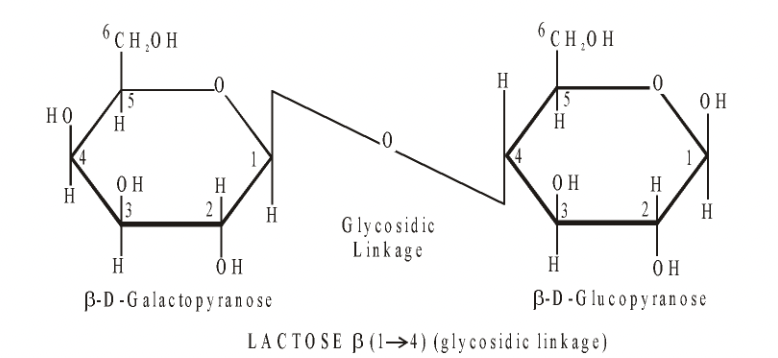
Fig. Structural formula of lactose
Sucrose = Glucose + Fructose
Cane sugar is most abundant disaccharide in nature, commonly found in plants, where it is transported in large quantities through phloem tissue. Also called Table sugar or commercial sugar.
Makes a good transport sugar because it is very soluble, and can therefore be moved efficiently in high concentrations and is also relatively unreactive chemically.
Obtained commercially from sugar cane and sugar beet and is the ‘sugar’ we normally buy in shops.
.png)
Fig. Structural formula of sucrose
Trehalose
A disaccharide sugar made of two glucose residues which was originally obtained from cocoon of Trehela (weevil Larinus species) as sweetening agent.
Found in haemolymph of some insects and fungi including yeast.
Trisaccharides : Made of three monosaccharide residues. A common triasaccharide is raffinose which is formed by condensation of galactose-glucose-fructose. It is a non reducing sugar which is found in some
plants as transport carbohydrate.
Tetrasaccharides : Formed by condensation of four monosaccharides. Trachyose is a nonreducing tetrasaccharide found in plants as transport carbohydrate. It is formed of galactose-galactose-glucose-fructose.
Reducing Sugars
All monosaccharides and some disaccharides, including maltose and lactose, are reducing sugars, meaning that they can carry out a type of chemical reaction known as reduction.
Sucrose is the only common non-reducing sugar.
Two common tests for reducing sugars, Benedict’s test and Fehling’s test make use of the ability of these sugars to reduce copper from a valency of 2 to a valency of 1. Both tests involve
use of an alkaline solution of copper (II) sulphate (CuSO4) which is reduced to insoluble copper (I) oxide (Cu2O).
Ionic equation : Cu2+ + e– → Cu+
Blue solution brick-red precipitate
POLYSACCHARIDES
Polymers of monosaccharides function chiefly as food and energy stores (for example starch and glycogen) and as structural materials (for example cellulose).
Convenient storage molecules for several reasons : their large size makes them more or less insoluble in water, so they exert no osmotic or chemical influence in the cell; they fold into compact shapes are easily converted to sugars by hydrolysis when required.
Structurally of two types :
Homopolysaccharides (homoglycans) : are complex carbohydrates that are formed by repeated condensation or polymerisation of only one type of monosaccharide monomers. e.g.
Pentosan - polymer of pentoses hexosan- polymer of hexoses, Araban- made of pentose arabinose, xylan- made of pentose xylose, glucan or glucosan- formed of glucose, fructan
orfructosan- formed of fructose, galactan or galactosan - formed of galactose.
Heteropolysaccharides (heteroglycans) : are complex carbohydrates that are formed by condensation of more than one type of monosaccharide monomers. Pectin, hemicellulose and
mucopolysaccharides are heteropolysaccharides made of two or more types of monosaccharide residues. Heteropolysaccharides and structural homopolysaccharides are functional
outside the cells of their formation. They come out of the cells wrapped in secretion vesicles of Golgi apparatus.
Functionally of three types:
MONOSACCHARIDES
Monosaccharides are single sugar units. Their general formula is (CH2O)n. They are classified according to the number of carbon atoms as trioses (3C), tetroses (4C), pentoses (5C),
hexoses (6C) and heptoses (7C). Of these, pentoses and hexoses are the most common.
Monosaccharides are important as energy sources and as building blocks for the synthesis of larger molecules.
Types :
1. Trioses (C3H6O3) e.g. Glyceraldehyde, Dihydroxyacetone. Intermediates in respiration (glycolysis), photosynthesis (dark reactions) and other kinds of carbohydrate metabolism.
2. Tetroses (C4H8O4) e.g. Erythrose, Threose, Erythrulose
Erythrose is an intermediate of respiratory and photosynthetic pathways and raw material for the synthesis of lignin, anthocyanins and some amino acids (like tyrosine and phenylalanine).
3. Pentoses (C5H15O5) e.g. ribose, deoxyribose (C5H10O4), xylose, arabinose, ribulose.
Synthesis of nucleic acid; ribose is a constituent of RNA, deoxyribose of DNA.
Synthesis of some coenzymes e.g. ribose used in the synthesis of NAD and NADP.
Ribulose biphosphate is the CO2 acceptor in photosynthesis.
Arabinose and xylose form polymers arabans and xylans which are constituents of hemicellulose.
4. Hexoses (C6H12O6) e.g. glucose, fructose, galactose, mannose.White, crystalline and sweet substances.
Glucose is blood sugar, a common respiratory substrate. Also called grape sugar, corn sugar and dextrose.
Fructose is sweetest naturally occuring sugar. Nectar and honey contain L form of fructose called Levulose. Also called fruit sugar.
5. Heptoses (C7H14O7) e.g. Sedoheptulose, Glucoheptose, Galactoheptose.
Sedoheptulose is intermediate of respiratory and photosynthetic pathways.
- Introduction of Cell Molecule
- Carbohydrates
- Structural Polysaccharides
- Sugar Part in Carbohydrates
- Storage Of Polysaccharides
- Lipids
- Constituents Of Lipids
- Types Of Lipids
- Amino Acid
- Protein
- Classification of Proteins
- Structure Of Proteins
- Denaturation and renaturation of proteins
- Nucleotides
- Structures of Nucleotides
- Formation of Nucleotides
- Vitamines of Nucleotides
- Nucleic Acid
- Minerals
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









