Constituents Of Lipids
Molecules of Cell of Class 11
Constituents of Lipids
Fatty acids
Fatty acids contain the acidic group –COOH (the carboxyl group) and are so named because some of the larger molecules in the series occur in fats.
They have the general formula R.COOH where R is hydrogen or a group such as –CH3, –C2H5, and so on, increasing by –CH2 for each subsequent member of the series.
There are usually many carbon atoms in the fatty acids used to make lipids. Most naturally occurring fatty acids have an even number of carbon atoms between 14 and 22 (most commonly
16 or 18). They have characteristically long chain of carbon and hydrogen atoms forming a hydrocarbon tail. Many of the properties of lipids are determined by these tails, including their
insolubility in water. The tails are said to be hydrophobic, meaning water-hating (hydro, water; phobos, fear).
Fatty acids sometimes contain one or more double bonds (C=C), such as oleic acid. In this case they are said to be unsaturated, (Cn H2n–2x O2), x for number of double bonds in chains as are lipids containing them.
Fatty acids and lipids lacking double bonds are said to be saturated (CnH2nO2).
Unsaturated fatty acids melt at much lower temperatures than saturated fatty acids. Oleic acid, for example, is the chief constituent of olive oil and is liquid at normal temperatures
(melting point 13.4 °C), whereas palmitic and stearic acids (melting points 63.1 °C and 69.6 °C respectively) are solid at normal body temperatures.
Alcohols
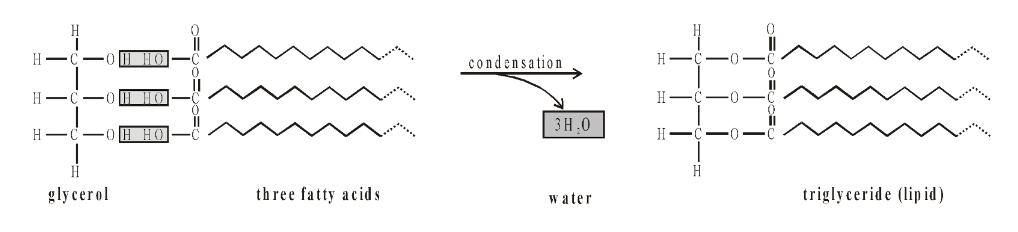
Most lipids are triglycerides. These are made from the alcohol glycerol. Glycerol has three hydroxyl (OH) groups, all of which can condense with a fatty acid.
- Introduction of Cell Molecule
- Carbohydrates
- Structural Polysaccharides
- Sugar Part in Carbohydrates
- Storage Of Polysaccharides
- Lipids
- Constituents Of Lipids
- Types Of Lipids
- Amino Acid
- Protein
- Classification of Proteins
- Structure Of Proteins
- Denaturation and renaturation of proteins
- Nucleotides
- Structures of Nucleotides
- Formation of Nucleotides
- Vitamines of Nucleotides
- Nucleic Acid
- Minerals
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









