Acyloin Condensation Reaction and Mechanism
Reactions and Introduction of Acyloin Condensation
When carboxylic acid esters are refluxed with metallic sodium in aprotic solvents such as ether, benzene, toluene or xylene. free from oxygen. a-hydroxy ketones called acyloins are formed. This is called acyloin condensation.

Mechanism of Acyloin Condensation
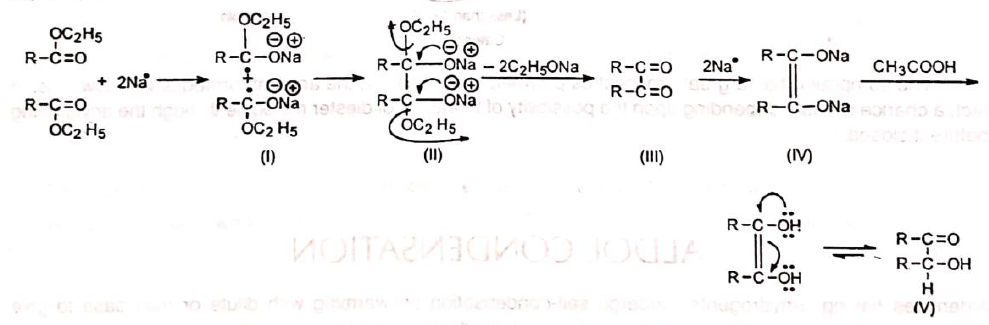
The mechanism of the condensation is not clearly known but it is observed that the reaction proceeds through a diketone intermediate, since diketone has been isolated in small amounts as a side product.As the reaction occurs in the presence of metallic sodium_ , a direct transfer of electron, i.e., a radical mechanism is suggested.The metallic sodium donates its electron to the carbonyl carbon to give (I) which subsequently dimerizes to yield (11). Loss of both the alkoxy groups from (II) produces 1, 2-diketone (Ill). Further reduction gives sodium salt of enediol (IV). Finally, addition of acid yields 1, 2-diol which tautomerizes to the stable acyloin (V).
Small traces of oxygen reduce the yield. Hence the reaction is carried out in an atmosphere of oxygen-free nitrogen.
Use of Acyloin Condensation
The condensation has considerable preparative value.
1.Acyloin Condensation is use in the Preparation of cyclic acyloins the condensation has been employed with great success for the preparation of _cyclic acyloin . Long-chain dicarboxylic esters have been converted to large-ring compounds without high dilution technique. The method 1s the best for closing rings of ten members or more.
The yields are as high as 60- 95% for 10 to 20 membered rings.
To account for the ready formation of large rings, it is suggested that the two ends of the ester are adsorbed, albeit weakly, to nearby sites on the surface of the sodium metal. Thus, the reactive ends are not
available for intermolecular coupling to compete with cyclisation. Do follow other Name reactions of organic chemistry prepared by Physics Wallah.
2.Preparation of catenane An interesting and unique compound called catenane, a compound witb interlocking rings, was formed when acyloin condensation was employed for ring closure with ester of 34-carbon dicarboxylic acid.
Recent Concepts
- Aldol condensation
- Arndt−Ester synthesis
- Baeyer−Villiger Oxidation
- Benzoin Condensation
- Beckmann Rearrangement
- Cannizzaro Reaction
- Clemmensen Reduction
- Claisen condensation
- Etard’s Reaction
- Friedel-Crafts alkylation
- Friedel Crafts Acylation
- Fries Rearrangement
- Gattermann-Koch Reaction
- Grignard Reagent
- Hell-Volhard-Zelinsky Reaction
- Hunsdieker reaction
- Hoffmann Bromamide Degradation
- Jones reagent
- Kolbes Reaction
- Knoevenagel Reaction









