Dienone phenol Rearrangements
About Dienone phenol Rearrangements
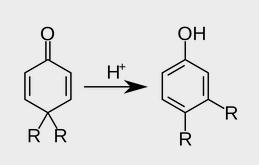
When 4, 4-dialkyl Cyclohexadienone is treated with acid, it is converted to phenol with migration of one of the alkyl groups to the adjacent carbon. This is known as dienone-phenol rearrangement. The dienone is dissolved in acetic anhydride and treated with catalytic amount of sulphuric acid. The product on hydrolysis gives the phenol.
Mechanism of Dienone phenol Rearrangements
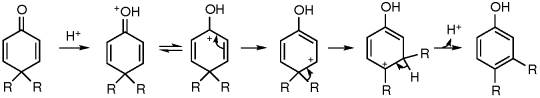
On protonation of the oxygen, a carbocation is generated' which is stabilized by de localization of the positive charge. In one of the canonical structures, the positive charge is on a carbon adjacent to a highly substituted carbon. Hence, a carbocation rearrangement occurs. Subsequent loss of a proton gives the 3, 4-disubstituted phenol. The ease of dienone-phenol rearrangement is due to the creation of a stable aromatic system.
When one of the alkyl group forms a part of the cyclic system, either the alkyl group or the ring methylene group may migrate. The course of the reaction depends on the structural or electronic factors and on the conditions of reaction. A reverse rearrangement, i.e., phenol--dienone rearrangement has been observed during the electrophilic substitution in phenols in some cases.
Applications of Dienone phenol Rearrangements
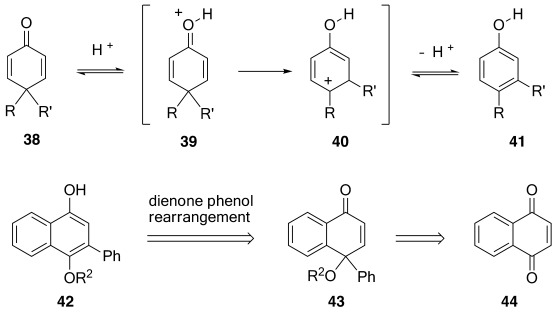
The rearrangement has useful applications. A classic example is the rearrangement of santonin to deisotope santonin.
Recent Concepts
- Aldol condensation
- Arndt−Ester synthesis
- Baeyer−Villiger Oxidation
- Benzoin Condensation
- Beckmann Rearrangement
- Cannizzaro Reaction
- Clemmensen Reduction
- Claisen condensation
- Etard’s Reaction
- Friedel-Crafts alkylation
- Friedel Crafts Acylation
- Fries Rearrangement
- Gattermann-Koch Reaction
- Grignard Reagent
- Hell-Volhard-Zelinsky Reaction
- Hunsdieker reaction
- Hoffmann Bromamide Degradation
- Jones reagent
- Kolbes Reaction
- Knoevenagel Reaction









