
Electromeric Effect
GOC of Class 11
Electromeric Effect
-
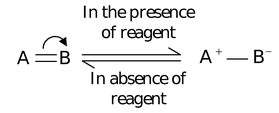
- Since A has lost its shared pair of electrons, it acquires a positive charge while B has gained the electron pair, thus it acquires a negative charge.
- The most common example illustrating the electromeric effect is the reaction of an alkene with Br2 in CCl4.
-
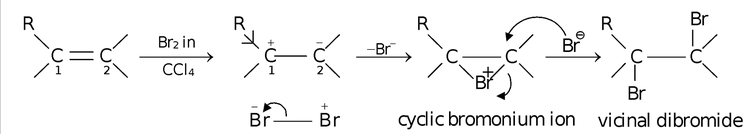
In this reaction, when reagent (bromine) approaches alkene, the temporary polarization develops on the alkene with C2 atom gaining a negative charge and C1 atom acquiring positive charge (as it can be compensated by the +I effect of R group). The alkenes being electron rich compounds (due to the presence of π−electron cloud) are attacked by the electrophile (Br+) to give a cyclic bromonium ion. Here, the formation of cyclic bromonium ion as intermediate is possible because bromine is of considerably large size having lone pairs to be bonded to both the carbons simultaneously. The cyclic bromonium ion is then attacked by Br− from the top (as lower side is already blocked) whereby the three membered ring is cleaved by trans opening giving vicinal dibromide as the product.
