Structural Isomerism
GOC of Class 11
Structural Isomerism
If the isomers have the same molecular formula but they differ in the relative arrangement of atoms, it is called structural isomerism. In structural isomers, the structural formula of the isomers differ whereas the molecular formula remains same. This type of isomerism is further divided into various types.
Chain or Nuclear Isomerism
This type of isomerism arises due to the difference in the structure of carbon chain. The difference may be in the length of the carbon chain or in the size of the carbon ring.
For example,
(i) n−butane and isobutane are chain isomers.
CH3−CH2−CH2−CH3 CH3−CH−CH3
|
n−butane CH3
isobutane
(ii) cyclohexane and methylcyclopentane are nuclear isomers.
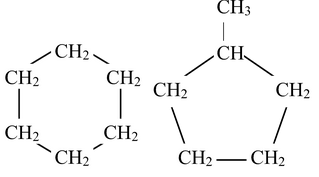
cyclohexane methylcyclopentane
(iii) C5H12 has three chain isomers.
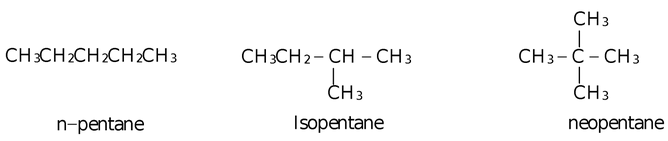
(iv) C4H9NH2 also shows two chain isomers.
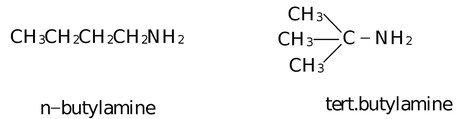
Positional Isomerism
This isomerism arises due to the difference in position of either substituent or functional group in the same carbon chain.
For example,
(i) CH3−CH2−CH = CH2 and CH3−CH = CH − CH3 are positional isomers.
1-Butene 2-Butene
(ii) CH3−CH2−CH2−Cl and CH3−CH−CH3
|
Cl
1−Chloropropane 2−Chloropropane are also positional isomers.
(iii) CH3−CH2−CH−CH2−CH3 and CH3−CH−CH2 − CH2−CH3
| |
CH3 CH3
3−Methylpentane 2−Methylpentane are positional isomers.
(iv) C6H4(NO2)2 exhibits following three positional isomers.
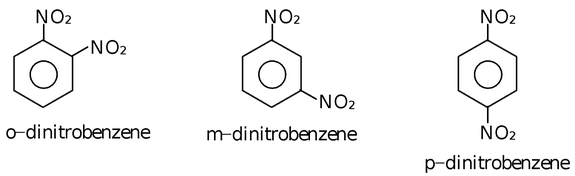
- Functional Isomerism
These isomers have same molecular formula but they differ only in the presence of different functional groups.
Carboxylic acids and esters are functional isomers. Alcohols and ethers are also functional isomers.
Aldehydes, ketones, unsaturated alcohols, unsaturated ethers, cyclic ethers and cyclic alcohols are functional isomers. Cyanides and Isocyanides are also functional isomers.
For example,
(i) C3H6O exhibits the following functional isomers.
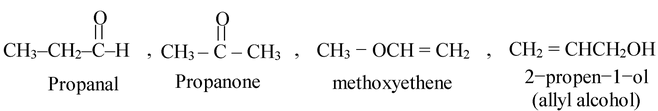
(ii) C3H6O2 shows following isomers.
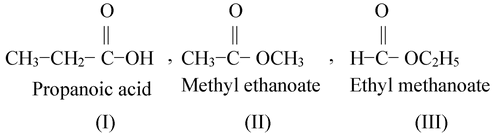
(I) & (II) and (I) & (III) are functional isomers.
(iii) C2H6O exhibits two functional isomers.
CH3−CH2−OH ; CH3−O−CH3
Ethanol Dimethyl ether
Tautomerism
This is a special type of functional isomerism where, functional isomers exist in equilibrium with each other. Such isomers are called tautomers. The necessary condition for this type of isomerism is the presence of hydrogen α− (a hydrogen on a carbon adjacent to carbon of functional group) to the carbonyl group.
A very common form of tautomerism is that between a carbonyl compound containing an α hydrogen and its enol form. This type of isomerism is also known as keto−enol isomerism.
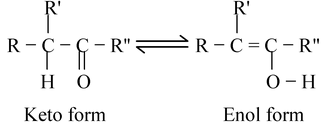
In simple cases (R" = H, alkyl, OR, etc) the equilibrium lies more towards the left. The reaction can be seen by examining the bond energies of various bonds. The keto form differs from the enol form in possessing a C − H, a C − C, and a C = O bond whereas the enol form has a C = C, a C − O and an O − H bond. The keto form is thermodynamically more stable than enol form and enol forms are normally not isolated. However in certain cases, a larger amount of the enol form is present, and it can even be the predominant form.
The percentage of enol form increases in the order simple aldehydes and ketones < β−keto esters < β−diketones < β−diketones having phenyl group < phenols. This increase in the enol content is due to the fact that the enol form of the above type of compounds is increasingly stabilized by resonance and hydrogen bonding than the corresponding keto form. There are four main types of the more stable enol forms.
(1) Molecules in which the enolic double bond is in conjugation with another double bond. For example in molecules like acetoacetic ester, the enol form is stabilized by internal hydrogen bonding, which is unavailable to the keto form.
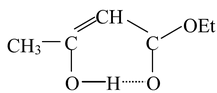
(2) Molecules that contain two or three bulky aryl groups. For example, in 2,2−dimesitylethenol, the keto content at equilibrium is only 5%.
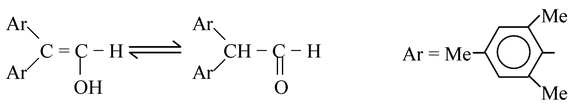
In such cases, the steric hindrance destabilizes the keto form. In the enol form, two aryl groups are about 120° apart, but in keto form they must move closer together (~109.5°). Such compounds are often called Fuson−type enols.
(3) Highly fluorinated enols are also stable than their corresponding keto form.
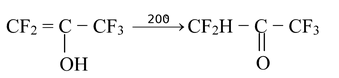
The enol form of such compounds can be kept at room temperature for long period of time becaue the tautomerization reaction is very slow, owing to the electron−withdrawing nature of the fluorines.
(4) The enol content would be more than the keto form if the former is stabilized by aromaticity. For example, phenol is an enol form.
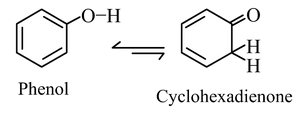
In those equilibria's, where the enol content is higher than the keto form, the solution produces red/green/blue colour with neutral FeCl3 solution. The appearance of such a colour on addition of neutral FeCl3 solution is a test for any enol. Phenol also responds to this test.
(Note: The tautomers are not counted while finding the total number of isomers for a given molecular formula)
Metamerism
It is caused by the attachment of different radicals/groups to a polyvalent atom (i.e. an atom having more than one valency). A metamer can be obtained by shifting one or more CH2 groups from one side of the polyvalent functional group to the other side. These isomers are of same homologous series. Metamerism is found to occur in amines, ethers, ketones, esters etc.
For example,
(i) C4H10O exhibits two metamers.
CH3−CH2−O−CH2−CH3 and CH3−CH2−CH2−O−CH3
Diethyl ether Methyl n−propyl ether
(ii) C5H10O2 exhibits following metamers.
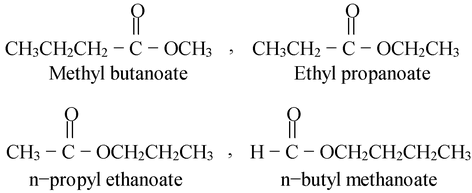
(iii) Similarly, C4H10NH exhibits following metamers.
C2H5 − NH − C2H5 CH3−NH−CH2CH2CH3
Diethyl amine N−methylpropyl amine
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









