Naming Of Cyclic Compound
GOC of Class 11
Naming Of Cyclic Compound
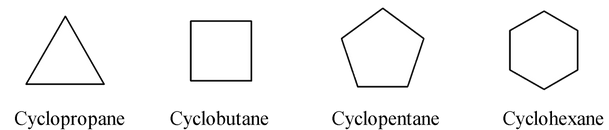
Hydrocarbon molecules with a ring but no π bonds are called cycloalkanes and have the molecular formula CnH2n. Molecules that have no rings are said to be acyclic. The IUPAC name for an cycloalkane is simply the name of the alkane with the same number of carbons, with the prefix “cyclo”. Thus the simplest cycloalkane is cyclopropane (C3H6) has a three membered ring. Cyclobutane (C4H8) has a four membered ring, cyclopentane has a five membered ring, and so on.
- In skeletal notation, cycloalkanes are usually drawn as regular polygons with the appropriate number of corners (one for each carbon).
-
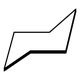
- But it is important to remember that, except for cyclopropane, these rings of carbon atoms are not completely planar. For example, the cyclohexane molecule actually prefers a lounge–like structure called the chair form, which looks like this
-
- To name a cycloalkane with only one substituent, simply add the substituent name as prefix to the cycloalkane root, as shown below
-
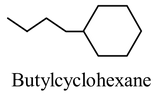
- It’s not necessary to number the location of a single susbstituent in a cycloalkane because all ring positions are equivalent.
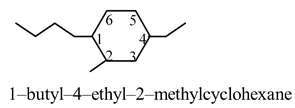
When there are two or more susbtituents, the one with the highest alphabetical priority is considered to be connected to the number–1 carbon of the ring. Counting around the ring is continued by the path that leads to the lowest sum of locator numbers. For example, in the structure below, the butyl group defines the number–1 carbon, C–1. We continue counting toward the next nearest substituent, the methyl group at C–2 followed by the ethyl group at C–4. However, in the name, ethyl appears before methyl. Thus we have 1–butyl–4–ethyl–2–methylcyclohexane
-
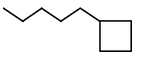
- You might be wondering how to name a structure that has a ring and a chain with the same number of carbons. The rule is this
- The ring determines the root name unless the chain has more carbons. In the latter case, the molecule is named as a derivative of the alkane, with a cycloalkyl substituent.
- For example, the structure below is 1–cyclobutylpentane, not pentylcyclobutane
-
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









